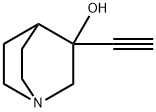
1-Azabicyclo[2.2.2]octan-3-ol, 3-ethynyl- (9CI) synthesis
- Product Name:1-Azabicyclo[2.2.2]octan-3-ol, 3-ethynyl- (9CI)
- CAS Number:19817-07-3
- Molecular formula:C9H13NO
- Molecular Weight:151.21
![1-Azabicyclo[2.2.2]octan-3-ol, 3-[2-(trimethylsilyl)ethynyl]-](/CAS/20210305/GIF/856677-58-2.gif)
856677-58-2
0 suppliers
inquiry
![1-Azabicyclo[2.2.2]octan-3-ol, 3-ethynyl- (9CI)](/CAS/GIF/19817-07-3.gif)
19817-07-3
20 suppliers
$350.00/1G
Yield:19817-07-3 43%
Reaction Conditions:
with potassium carbonate in methanol; for 3 h;
Steps:
3-Ethynyl-3-hydroxyquinuclidine (67)
3-Ethynyl-3-hydroxyquinuclidine (67)
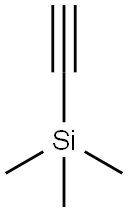
A solution of ethynyl(trimethyl)silane 65 (4.44 mL, 31.4 mmol) in 30 mL of THF was cooled to -10° C. and then 12.6 mL of a solution of n-BuLi (2.5 M in hexane) was added therein drop by drop.
After 10 minutes of stirring at -10° C., the reaction medium was cooled to -78° C. and a solution of 3-quinuclidone 64 (3.74 g, 29.9 mmol) in 70 mL of THF was added therein.
Upon completion of the addition, the cold bath was removed and the reaction mixture was allowed to return to ambient temperature.
After an additional hour of reaction, it was hydrolysed with a saturated NaCl solution.
The two phases were separated and the aqueous phase was extracted with ethyl acetate.
The organic phases were combined, dried over anhydrous MgSO4, and filtered and evaporated under reduced pressure.
The silyl derivative 66 (28.1 mmol) thus prepared was directly engaged in the deprotection reaction by means of stirring for a period of 3 hours in 60 ml of methanol in the presence K2CO3 (3.89 g, 28.1 mmol).
The reaction medium was evaporated under reduced pressure and the residue was purified by column chromatography on silica gel with the eluent used being a mixture of CH2Cl2/MeOH/NH4OH (80/20/0.1).
The product 67 was isolated in the form of a white solid with a yield of 43%. Rf: 0.35 (CH2Cl2/MeOH/NH4OH: 80/20/0.1); Mp: 201° C.; IR (ATR, Diamond): ν (cm-1): 989, 1024, 1048, 1071, 1138, 1154, 1317, 1455, 2596, 2754, 2873, 2934, 2950, 2965, 3216; 1H NMR (250 MHz, MeOD): δ (ppm) 1.38-1.53 (m, 1H), 1.61-1.75 (m, 1H), 1.93-2.10 (m, 3H), 2.73-2.84 (m, 4H), 2.88 (d, 1H, J=13.8 Hz), 2.89 (s, 1H), 3.13 (d, 1H, J=13.8 Hz); 13C NMR (100 MHz, MeOD): δ (ppm) 20 3 (CH2), 24.2 (CH2), 34.2 (CH), 46.9 (CH2), 47.0 (CH2), 65.0 (CH2), 67.4 (Cq); MS (IS): m/z=152.8 [MH]+.
References:
US2014/30191,2014,A1 Location in patent:Paragraph 0561; 0562; 0563
![Butanoic acid, 3-ethynyl-1-azabicyclo[2.2.2]oct-3-yl ester](/CAS/20210305/GIF/160127-73-1.gif)
160127-73-1
0 suppliers
inquiry
![1-Azabicyclo[2.2.2]octan-3-ol, 3-ethynyl- (9CI)](/CAS/GIF/19817-07-3.gif)
19817-07-3
20 suppliers
$350.00/1G
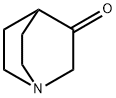
3731-38-2
88 suppliers
inquiry
![1-Azabicyclo[2.2.2]octan-3-ol, 3-ethynyl- (9CI)](/CAS/GIF/19817-07-3.gif)
19817-07-3
20 suppliers
$350.00/1G
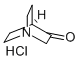
1193-65-3
460 suppliers
$19.00/5g
![1-Azabicyclo[2.2.2]octan-3-ol, 3-ethynyl- (9CI)](/CAS/GIF/19817-07-3.gif)
19817-07-3
20 suppliers
$350.00/1G