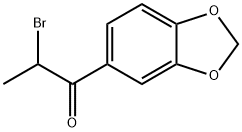
2-Bromo-3',4'-(methylenedioxy)propiophenone synthesis
- Product Name:2-Bromo-3',4'-(methylenedioxy)propiophenone
- CAS Number:52190-28-0
- Molecular formula:C10H9BrO3
- Molecular Weight:257.08
![1-(benzo[d][1,3]dioxol-5-yl)-2-bromopropan-1-one synthesis 1-(benzo[d][1,3]dioxol-5-yl)-2-bromopropan-1-one synthesis](/NewsImg/2023-05-08/6381915544832604198489645.png)
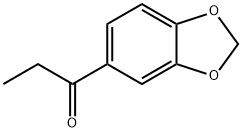
28281-49-4
158 suppliers
$89.09/5g

52190-28-0
85 suppliers
$140.00/50mg
Yield:52190-28-0 87%
Reaction Conditions:
with bromine in chloroform at 0;
Steps:
4.1.8 4.1.8 1-(4-Benzyloxy-3-methoxyphenyl)-2-bromopropan-1-one (5a)
General procedure: To a solution of 4a (3.90?g, 14.4?mmol) in CHCl3 (100?mL) was added Br2 (741?μL, 14.4?mmol) in CHCl3 (45.0?mL) at 0?°C. After being stirred overnight, saturated NaHCO3 solution was added and extracted with DCM. The organic layers were dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography (hexane:EtOAc?=?88:12) to afford 5a (3.93?g, 78%) as a colorless oil.
References:
Harada, Kenichi;Zaha, Katsuyoshi;Bando, Rina;Irimaziri, Ryo;Kubo, Miwa;Koriyama, Yoshiki;Fukuyama, Yoshiyasu [European Journal of Medicinal Chemistry,2018,vol. 148,p. 86 - 94]
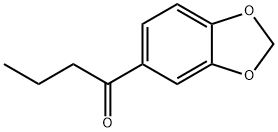
63740-97-6
83 suppliers
$8.00/1g

52190-28-0
85 suppliers
$140.00/50mg
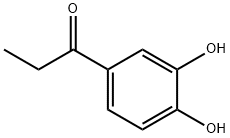
7451-98-1
66 suppliers
$42.32/1g

52190-28-0
85 suppliers
$140.00/50mg