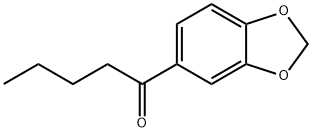
1-(benzo[d][1,3]dioxol-5-yl)pentan-1-one synthesis
- Product Name:1-(benzo[d][1,3]dioxol-5-yl)pentan-1-one
- CAS Number:63740-98-7
- Molecular formula:C12H14O3
- Molecular Weight:206.24

109-65-9
546 suppliers
$10.00/10g
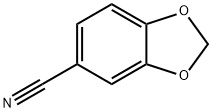
4421-09-4
153 suppliers
$8.00/1g
![1-(benzo[d][1,3]dioxol-5-yl)pentan-1-one](/CAS/GIF/63740-98-7.gif)
63740-98-7
65 suppliers
$34.00/250mg
Yield:63740-98-7 68%
Reaction Conditions:
Stage #1: 1-bromo-butanewith magnesium in diethyl ether; for 3 h;Inert atmosphere;Reflux;
Stage #2: piperonylonitrile in toluene; for 4 h;Reflux;
Steps:
1; 2 Example 1: Synthesis of bromobutane magnesium reagent
250 ml dry four-neck bottle,Install a nitrogen snorkel,A reflux condenser with an anhydrous calcium chloride drying tube and a constant pressure dropping funnel with an anhydrous calcium chloride drying tube were inserted at the upper end.The funnel was charged with 40 ml of absolute dry diethyl ether and 7.23 g of 1-bromobutane.Add 40 ml of absolute dry ether to the four-neck bottle.1.15 grams of magnesium metal shavings and two iodine particles.Start stirring,After nitrogen replacement,Add the 1-bromobutane ether solution to the four-neck bottle.After half an hour, the addition was completed, and the temperature was refluxed for 3 hours.Then the temperature is lowered to room temperature.;5.89 g of 3,4-methylenedioxybenzonitrile and 50 ml of toluene were added from the constant pressure dropping funnel to the Grignard reagent prepared in the previous step.The addition was completed in 1 hour.After evaporating the ether at elevated temperature,Add 30 ml of toluene,Warming reflux reaction,The progress of the reaction was monitored by thin layer chromatography.After the reflux reaction for 3 hours, the reaction was completed.Down to room temperature,Add 40 ml of ice water mixture and 8 ml of concentrated sulfuric acid.After the hydrolysis reaction,The liquid phase was separated and the aqueous phase was extracted twice with 100 ml of diethyl ether.Combine the organic phase,Wash once with 100 ml of saturated saline solution.Wash with deionized water twice,It was dried overnight by adding anhydrous sodium sulfate.The solvent was removed by rotary evaporation to give a crude oil.This crude product was separated by flash chromatography (silica gel eluent: ethyl acetate: petroleum ether = 1:9) to give 6.55 g (yield 68%) of object.1-(Benzo[D][1,3]dioxol-5-yl)pentan-1-one.
References:
CN108276392,2018,A Location in patent:Paragraph 0082; 0083; 0085
274-09-9
458 suppliers
$5.00/5g
638-29-9
262 suppliers
$14.14/1gm:
![1-(benzo[d][1,3]dioxol-5-yl)pentan-1-one](/CAS/GIF/63740-98-7.gif)
63740-98-7
65 suppliers
$34.00/250mg
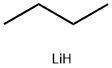
29786-93-4
0 suppliers
inquiry

124-38-9
126 suppliers
$214.00/14L

17680-04-5
30 suppliers
$210.00/100ml
![1-(benzo[d][1,3]dioxol-5-yl)pentan-1-one](/CAS/GIF/63740-98-7.gif)
63740-98-7
65 suppliers
$34.00/250mg
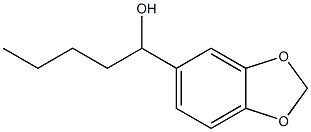
5422-01-5
11 suppliers
inquiry
![1-(benzo[d][1,3]dioxol-5-yl)pentan-1-one](/CAS/GIF/63740-98-7.gif)
63740-98-7
65 suppliers
$34.00/250mg
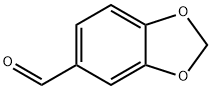
120-57-0
5 suppliers
$26.35/25g
![1-(benzo[d][1,3]dioxol-5-yl)pentan-1-one](/CAS/GIF/63740-98-7.gif)
63740-98-7
65 suppliers
$34.00/250mg