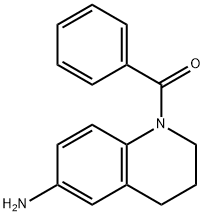
1-benzoyl-1,2,3,4-tetrahydroquinolin-6-amine synthesis
- Product Name:1-benzoyl-1,2,3,4-tetrahydroquinolin-6-amine
- CAS Number:857759-22-9
- Molecular formula:C16H16N2O
- Molecular Weight:252.31
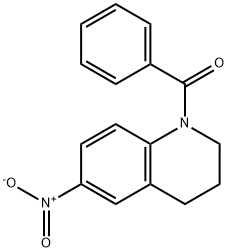
30450-57-8
0 suppliers
inquiry

857759-22-9
8 suppliers
inquiry
Yield:857759-22-9 79%
Reaction Conditions:
with ammonium chloride;zinc in methanol;water at 60; for 6 h;
Steps:
5.7. Synthesis of (6-amino-3,4-dihydroquinolin-1(2H)-yl)(4-chlorophenyl)methanone (8a):
General procedure: The mixture of ammonium chloride (7000 mmol, 0.5 gm) was preparedin water: methanol (3:7). Simultaneously the compound 7a (1000mmol, 0.3 gm) was added to suitable quantity of methanol followed bythe addition of the zinc dust (10000 mmol, 0.43 gm). The above mixtureof ammonium chloride was poured into this reaction mixture of compoundand zinc dust. This reaction mixture was allowed to stir at 60 Cfor 6 h resulted into the reduction of nitro group to amine. Furthermethanol was evaporated and the mixture was dissolved in the ethylacetate and extracted with bicarbonate. The compound was dried overthe sodium sulfate. Yield: 80%; mp: 190-193 C; IR (KBr, γmax cm 1):1552(C- -O), 1600(C- -C), 2360(C-N), 2931(C-H aliphatic), 3361(C-H aromatic); MS (EI) m/z calculated for C16H15ClN2O 287 (M + 1),found 287.5 (M + 1) and 289.5 for chloro pattern.
References:
Chaube, Udit J.;Rawal, Rakesh;Jha, Abhishek B.;Variya, Bhavesh;Bhatt, Hardik G. [Bioorganic Chemistry,2021,vol. 106]
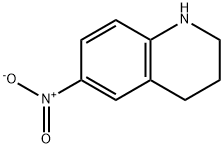
14026-45-0
86 suppliers
inquiry

857759-22-9
8 suppliers
inquiry

98-88-4
576 suppliers
$10.00/5g

857759-22-9
8 suppliers
inquiry
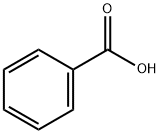
65-85-0
1334 suppliers
$10.00/25g

857759-22-9
8 suppliers
inquiry
613-50-3
240 suppliers
$10.00/5g

857759-22-9
8 suppliers
inquiry