
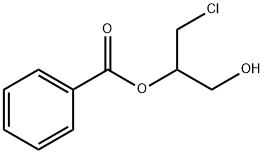
1-Benzoyloxy-3-chloropropan-2-ol synthesis
- Product Name:1-Benzoyloxy-3-chloropropan-2-ol
- CAS Number:3477-94-9
- Molecular formula:C10H11ClO3
- Molecular Weight:214.65
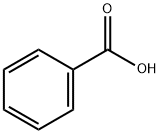
65-85-0
1341 suppliers
$10.00/25g
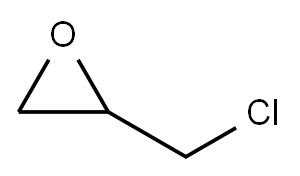
106-89-8
737 suppliers
$9.00/10g

3477-94-9
4 suppliers
inquiry
36847-79-7
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with tetrabutylammomium bromide in ethyl acetate at 65; for 4 h;Overall yield = 87 percent; Overall yield = 3.58 g; regioselective reaction;
Steps:
Ring opening in epichlorohydrin (general procedure).
General procedure: Epichlorohydrin (21.2 mmol) and TBAB(5 mol %) were added to a solution of carboxylic acid (19.2 mmol) in EtOAc. The reaction mixture was refl uxed at 65°C for 4 h and then cooled down to room temperature and washed with a saturated Na2CO3 solution. The organic layer was dried over anhydrous Na2SO4 and evaporated to dryness, and the product was purifi ed by column chromatography on silica gel, eluentpetroleum ether-ethyl acetate, 5 : 10.
References:
Feng, Y.;He, W.;Luo, Y.;Sun, W.;Xia, X. Y.;Zhan, L. [Russian Journal of Organic Chemistry,2020,vol. 56,# 5,p. 877 - 883][Zh. Org. Khim.,2020,vol. 56,# 5,p. 877 - 883,7]

![4-CHLOROMETHYL-2-PHENYL-[1,3]DIOXOLANE](/StructureFile/ChemBookStructure4/GIF/CB8678113.gif)