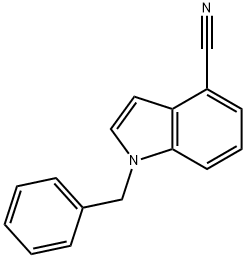
1-benzyl-1H-indole-4-carbonitrile(SALTDATA: FREE) synthesis
- Product Name:1-benzyl-1H-indole-4-carbonitrile(SALTDATA: FREE)
- CAS Number:177548-00-4
- Molecular formula:C16H12N2
- Molecular Weight:232.28
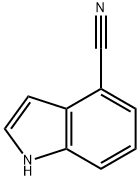
16136-52-0
246 suppliers
$10.00/1g

100-39-0
425 suppliers
$10.00/10g

177548-00-4
12 suppliers
inquiry
Yield:177548-00-4 99%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 20;Inert atmosphere;
Steps:
G.i
To a solution of indole-4-carbonitrile (2.0g, 14.1 mmol, 1 eq) and benzyl bromide (2.17ml_, 18.3mmol, 1.3eq) in dry DMF (20ml_) was added, at rt, NaH (733mg, 18.3mmol, 1 .3eq) portionwise under Ar(g). The resulting reaction mixture was stirred at rt overnight, and was subsequently partitioned with H20 (30ml_), and then extracted with EtOAc (3 x 20ml_) and CH2CI2 (20ml_). The combined organic extracts were dried over MgS04 and the solvent was removed in vacuo. The residue was further purified by silica gel column chromatography with hexane/EtOAc (9:1-1 :3) to furnish the product, 2, as a white solid (3.30g, 99%). 1H NMR (400MHz, CDCI3) δΗ: 7.49 (t, J=8.6 Hz, 2H), 7.29-7.36 (m, 4H), 7.18-7.23 (m, 1 H), 7.07-7.13 (m, 2H), 6.75-6.80 (m, 1 H), 5.38 (s, 2H).MS (ES+) 255.2 (100%, [M+Na]+).
References:
WO2011/135351,2011,A1 Location in patent:Page/Page column 26

16136-52-0
246 suppliers
$10.00/1g

177548-00-4
12 suppliers
inquiry