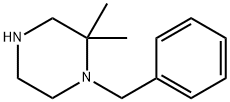
1-benzyl-2,2-dimethylpiperazine synthesis
- Product Name:1-benzyl-2,2-dimethylpiperazine
- CAS Number:846052-91-3
- Molecular formula:C13H20N2
- Molecular Weight:204.31
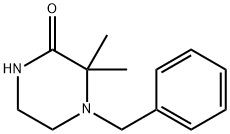
29906-46-5
6 suppliers
inquiry

846052-91-3
7 suppliers
inquiry
Yield:846052-91-3 5.2 g
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 25; for 6 h;Reflux;
Steps:
2.2 Step 2: 1 -benzyl-2 2-dimethylpiperazine.
To a stirred solution of lithiulalluminium hydride (1.078 g, 28.4 mmol) in tetrahudrofuran (200 ml) at 0-20 °C was added 4-benzyl-3,3-dimethylpiperazin- 2-one (6.2 g, 28.4 mmol in 100 ml tetrahydrofuran) over lh. The reaction mixture was warmed to 25 °C over 15 minutes followed by reflux for 6h. Theprogress of reaction was monitored by TLC. After completion of reaction, the reaction mixture was cooled to 0-10 °C and 10% sodium hydroxide (50 ml) was added slowly over lh. and then allowed to stirr for 20 hr at 25 °C. The reaction mass was passed through celite and washed with tetrahydrofuran (500m1). The combined flitrate was concentrated under reduced pressure. Water (100 ml) was added and the aq. phase was extracted with ethyl acetate (3 x 100m1). The combined organic layer was washed with brine (50 ml) and dried over sodium sulphate, and concentrated under reduced pressure till dryness. The resultingcrude product (5.2 g) was carried over to the next step without purification.‘H NMR (400 MHz, CDC13) 6 7.37-7.21(m, 5H), 3.53 (bs, 1K D20 exchangeable), 3.52 (s, 2H), 2.82-2.80 (m, 2H), 2.69 (s, 2H), 2.37-2.31(m, 2H), 1.17 (s, 6H).
References:
WO2014/9872,2014,A1 Location in patent:Page/Page column 61; 62

22476-74-0
86 suppliers
$14.00/250mg

846052-91-3
7 suppliers
inquiry

100-39-0
425 suppliers
$10.00/10g

846052-91-3
7 suppliers
inquiry