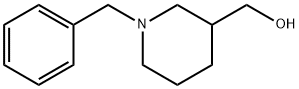
1-BENZYL-3-HYDROXYMETHYLPIPERIDINE synthesis
- Product Name:1-BENZYL-3-HYDROXYMETHYLPIPERIDINE
- CAS Number:85387-44-6
- Molecular formula:C13H19NO
- Molecular Weight:205.3
72551-53-2
83 suppliers
$8.00/5g

85387-44-6
41 suppliers
$60.00/100mg
Yield:85387-44-6 99%
Reaction Conditions:
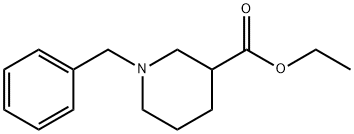
Stage #1: ethyl 1-benzylpiperidine-3-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at 5; for 1 h;
Stage #2: with ethyl acetate in tetrahydrofuran;
Steps:
A5
(l-Benzylpipeiidin-3-yl)methanol; To a solution of ethyl l-benzylpiperidine-3-carboxylate (1.0 g) in tetrahydrofuran (10 mL) at 5°C under argon was added a IM solution of lithium aluminium hydride (4.04 mL) dropwise. The mixture was stirred for 1 hour at 5°C and then quenched by the dropwise addition of ethyl acetate (5 mL). The mixture was warmed to room temperature and dichloromethane (150 mL) was added followed by saturated aqueous sodium potassium tartrate (50 mL). The mixture was stirred vigorously overnight. The layers were separated EPO
References:
WO2006/67401,2006,A1 Location in patent:Page/Page column 190-191
4606-65-9
248 suppliers
$9.00/1g

100-39-0
425 suppliers
$10.00/10g

85387-44-6
41 suppliers
$60.00/100mg

4606-65-9
248 suppliers
$9.00/1g

100-52-7
943 suppliers
$20.37/250g

85387-44-6
41 suppliers
$60.00/100mg

614-18-6
461 suppliers
$5.00/10g

85387-44-6
41 suppliers
$60.00/100mg

4606-65-9
248 suppliers
$9.00/1g

100-44-7
630 suppliers
$13.50/250G

85387-44-6
41 suppliers
$60.00/100mg