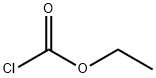
1-BENZYL-4-HYDROXY-5-AZAINDOLE synthesis
- Product Name:1-BENZYL-4-HYDROXY-5-AZAINDOLE
- CAS Number:26956-47-8
- Molecular formula:C14H12N2O
- Molecular Weight:224.26
![2-Propenoyl azide, 3-[1-(phenylmethyl)-1H-pyrrol-2-yl]-, (2E)-](/CAS/20211123/GIF/60290-15-5.gif)
60290-15-5
0 suppliers
inquiry

26956-47-8
21 suppliers
inquiry
Yield:26956-47-8 81%
Reaction Conditions:
with tributyl-amine in diphenylether;4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran at 195; for 1 h;
Steps:
INTERMEDIATE 89 [1-BENZYL-1,] 5-dihydro-pyrrolo [3, 2-c] pyridin-4-one
INTERMEDIATE 89 [1-BENZYL-1,] 5-dihydro-pyrrolo [3, 2-c] pyridin-4-one was prepared by the literature procedure according to C. Ducrocq; E. Bisangi; J-M, Lhoste; J. Mispelter; Tetrahedron, Vol 32, pp 773-780, (1976). To a stirred solution [OF N-TRIBUTYLAMINE] (30 mL) in diphenyl ether (150 mL) heated to 195 [°C] was slowly added during 30 minutes a solution of the acyl azide dissolved in DCM (150 mL). The reaction mixture was stirred at [195 °C] for 1 hour and then cooled to room temperature. Pentane (1.0 L) and ether (1.0 L) was added to the reaction mixture and the precipitate was collected by filtration. The crude solid was triturated with ether to give 6.89 g [(81 %)] of the pure product. Purity HPLC >95%; MS [(ESI)] m/z 225 (m+H) [; 1H] NMR (DMSO-d6, [25 °C,] 270.16) 8 10.84 (br s, 1 H), 7.43-7. 14 (m, 6 H), 7.00 (d, J = 7.12 Hz, 1 H), 6.57-6. 49 (m, 2 H), 5.83 (s, 2 H).
References:
WO2004/828,2003,A1
![3-[2-(N-BENZYL)PYRROLYL] ACRYLIC ACID](/CAS/GIF/60290-03-1.gif)
60290-03-1
4 suppliers
inquiry

26956-47-8
21 suppliers
inquiry

18159-24-5
22 suppliers
inquiry

26956-47-8
21 suppliers
inquiry