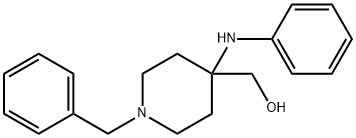
1-benzyl-4-(phenylamino)piperidine-4-methanol synthesis
- Product Name:1-benzyl-4-(phenylamino)piperidine-4-methanol
- CAS Number:61086-04-2
- Molecular formula:C19H24N2O
- Molecular Weight:296.41

61085-60-7
39 suppliers
$281.00/1g

61086-04-2
22 suppliers
$110.09/10mg
Yield:61086-04-2 98%
Reaction Conditions:
Stage #1: methyl 4-(phenylamino)-1-(phenylmethyl)-4-piperidinecarboxylatewith sodium bis(2-methoxyethoxy)aluminium dihydride in toluene at 20; for 2.25 h;
Stage #2: with sodium hydroxide;water in toluene at 25 - 30;
Steps:
5
Example 5; Synthesis of l-Benzyl-4-phenylamino-4-(hydroxymethyl) piperidine:; A 3.0 L, 4- necked R.B flask was charged with 84.0 g (0.26 mol) of the methyl ester produced in Example 4 followed by 420 mL of toluene under a nitrogen atmosphere. The mixture was stirred to obtain a colorless clear solution. 157.0 g (0.77 mol) of the Red-Al, vitride was added drop wise to the stirred solution over a period of 35 minutes. After the addition was complete, stirring continued at room temperature for and 100 minutes. Then, a 5% NaOH solution (175 mL) was added drop wise very slowly keeping the internal temperature in the range of 25-30 0C. The reaction mixture then was transferred to a separatory funnel and the bottom milky layer was separated. The organic layer was washed with brine (100 mL) and then dried over Na2SO4. The solution was filtered and toluene was removed under reduced pressure and the resulting oil was dried in vacuuo to get 82.1 g of viscous and light yellow oil (98%, corrected for residual solvents). Purity of Compound 6 was determined to be 99% by liquid chromatography. 1H NMR (CDCI3): δ 7.5-6.6(m, 10H), 3.6(s, 2H)5 3.5(s, 2H)5 2.6(m,3H), 2.3(t, 2H)5 1.9(d, 2H)5 1.6(dt, 2H).
References:
WO2008/5423,2008,A1 Location in patent:Page/Page column 3; 8
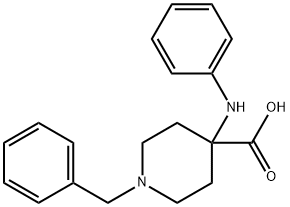
85098-64-2
75 suppliers
$13.00/100mg

61086-04-2
22 suppliers
$110.09/10mg
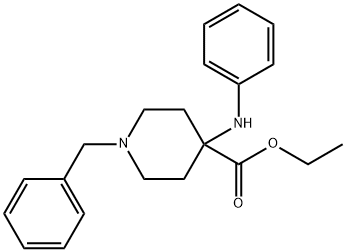
63260-82-2
1 suppliers
inquiry

61086-04-2
22 suppliers
$110.09/10mg

61085-60-7
39 suppliers
$281.00/1g
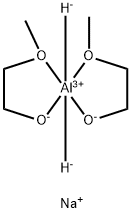
22722-98-1
244 suppliers
$22.00/25g

61086-04-2
22 suppliers
$110.09/10mg

63260-82-2
1 suppliers
inquiry

22722-98-1
244 suppliers
$22.00/25g

61086-04-2
22 suppliers
$110.09/10mg