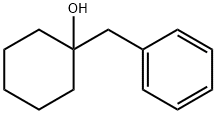
1-benzylcyclohexan-1-ol synthesis
- Product Name:1-benzylcyclohexan-1-ol
- CAS Number:1944-01-0
- Molecular formula:C13H18O
- Molecular Weight:190.28

108-94-1
528 suppliers
$12.00/50g

100-39-0
422 suppliers
$10.00/10g

1944-01-0
14 suppliers
$200.00/250mg
Yield:1944-01-0 90%
Reaction Conditions:
Stage #1: benzyl bromidewith magnesium in tetrahydrofuran at 60; for 1 h;
Stage #2: cyclohexanone in tetrahydrofuran; for 8 h;Reflux;
Steps:
3 Preparation of 1-benzylcyclohexan-1-ol
171 g (1 mol) of benzyl bromide was slowly added into 36.5 g (1.5 mol) of magnesium powder in 500 g of dry THF to form a first mixture.
The first mixture was gently heated to 60° C. and stirred for 1 hour, and then 117.8 g (1.2 mol) of anhydrous cyclohexanone was slowly added to form a second mixture.
The second mixture was heated to reflux for 8 hours.
After cooling to room temperature, 1000 g of 10% hydrochloric acid was added, and the resulting mixture was extracted with 500 g of dichloromethane twice, Upon separation of the phases, the organic layer was dried with a desiccant (magnesium sulfate).
A colorless liquid, 1-benzylcyclohexan-1-ol, with a yield of 90% was obtained after the removal of desiccant and solvent from the organic layer.
References:
US2017/36981,2017,A1 Location in patent:Paragraph 0160; 0161

770-09-2
115 suppliers
$35.00/5mL

108-94-1
528 suppliers
$12.00/50g

1944-01-0
14 suppliers
$200.00/250mg

108-94-1
528 suppliers
$12.00/50g
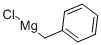
6921-34-2
219 suppliers
$74.79/50ml

1944-01-0
14 suppliers
$200.00/250mg

108-94-1
528 suppliers
$12.00/50g

100-44-7
626 suppliers
$13.50/250G

1944-01-0
14 suppliers
$200.00/250mg
5281-18-5
44 suppliers
$75.00/50mg

108-94-1
528 suppliers
$12.00/50g

1944-01-0
14 suppliers
$200.00/250mg