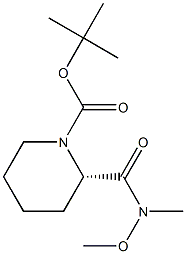
1-BOC-(2S)-[N-METHOXY-N-METHYLCARBAMOYL]PIPERIDINE synthesis
- Product Name:1-BOC-(2S)-[N-METHOXY-N-METHYLCARBAMOYL]PIPERIDINE
- CAS Number:203056-27-3
- Molecular formula:C13H24N2O4
- Molecular Weight:272.34

6638-79-5
553 suppliers
$6.00/25g
26250-84-0
287 suppliers
$9.00/5g
![1-BOC-(2S)-[N-METHOXY-N-METHYLCARBAMOYL]PIPERIDINE](/CAS/GIF/203056-27-3.gif)
203056-27-3
18 suppliers
$110.00/1g
Yield:203056-27-3 94%
Reaction Conditions:
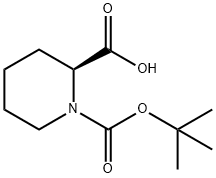
Stage #1: (S)-(-)-1-(tert-butoxycarbonyl)-2-piperidinecarboxylic acidwith 1,1'-carbonyldiimidazole in dichloromethane at 20;
Stage #2: N,O-dimethylhydroxylamine*hydrochloride in dichloromethane at 20; for 17 h;
Steps:
1.1 (S)-1-(tert-butoxycarbonyl)-2-(N-methyl-N-methoxy)-carbamoylpiperidine 2:
Dissolve 20.0g (87.2mmol) (S)-1-(tert-butoxycarbonyl)-2-piperidinecarboxylic acid 1 in 400mL dichloromethane,slowly add 18.4g (113.5 mmol) N,N’-carbonyldiimidazole,stir at room temperature until no bubbles emerge,add 11.6g (113.4mmol) N,O-dimethylhydroxylamine hydrochloride,stir at room temperature for 17 hours.after the reaction mixture was diluted with 150 mL of water,extract with dichloromethane (60mL×3),Dry the organic phase with anhydrous sodium sulfate,filter,remove the solvent under reduced pressure,column chromatography separation,V (petroleum ether): V (ethyl acetate) = 4:1,22.3 g of colorless oily liquid was obtained, and the yield was 94%.
References:
CN111689957,2020,A Location in patent:Paragraph 0038-0042

26250-84-0
287 suppliers
$9.00/5g

1117-97-1
69 suppliers
inquiry
![1-BOC-(2S)-[N-METHOXY-N-METHYLCARBAMOYL]PIPERIDINE](/CAS/GIF/203056-27-3.gif)
203056-27-3
18 suppliers
$110.00/1g

6638-79-5
553 suppliers
$6.00/25g
![1-BOC-(2S)-[N-METHOXY-N-METHYLCARBAMOYL]PIPERIDINE](/CAS/GIF/203056-27-3.gif)
203056-27-3
18 suppliers
$110.00/1g

24424-99-5
821 suppliers
$13.50/25G
![1-BOC-(2S)-[N-METHOXY-N-METHYLCARBAMOYL]PIPERIDINE](/CAS/GIF/203056-27-3.gif)
203056-27-3
18 suppliers
$110.00/1g
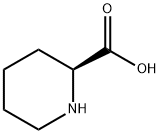
3105-95-1
368 suppliers
$5.00/100mg
![1-BOC-(2S)-[N-METHOXY-N-METHYLCARBAMOYL]PIPERIDINE](/CAS/GIF/203056-27-3.gif)
203056-27-3
18 suppliers
$110.00/1g