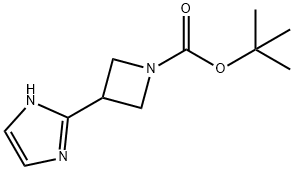
1-boc-3-(1h-iMidazol-2-yl)azetidine synthesis
- Product Name:1-boc-3-(1h-iMidazol-2-yl)azetidine
- CAS Number:1234710-02-1
- Molecular formula:C11H17N3O2
- Molecular Weight:223.27
177947-96-5
169 suppliers
$11.00/100mg

131543-46-9
0 suppliers
inquiry

1234710-02-1
19 suppliers
$200.00/25mg
Yield:1234710-02-1 42%
Reaction Conditions:
with ammonia at 0 - 26; for 14.1667 h;
Steps:
95.1 Step 1: Preparation of tert-butyl 3-(1H-imidazol-2-yl)azetidine-1-carboxylate
Step 1:
Preparation of tert-butyl 3-(1H-imidazol-2-yl)azetidine-1-carboxylate
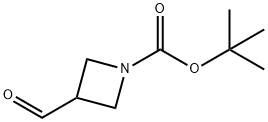
Ammonia gas was bubbled through a mixture of tert-butyl 3-formylazetidine-1-carboxylate (1.0 g, 5.4 mmol, 1.0 eq) and glyoxal (10.9 g, 40 wt % in water, 75.59 mmol, 14 eq.) at 0° C. for 10 min, until the weight of the solution increase 1.84 g (about 107.98 mmol of NH3).
The mixture was allowed to warm to 26° C. and stirred for 14 hours.
The aqueous layers were extracted with CH2Cl2.
The combined organic phase was dried over Na2SO4, filtered and concentrated under reduced pressure.
The residue was purified by silica gel chromatography eluting with 70% EtOAc in hexane to afford the title compound (0.51 g, 42% yield) as a light yellow solid. MS (ESI) m/z 224.0 [M+H]+.
1H NMR (400 MHz, CDCl3) δ 7.01 (s, 2H), 4.28 (t, J=8.8 Hz, 2H), 4.16-4.11 (m, 2H), 3.88-3.86 (m, 1H), 1.44 (s, 9H).
References:
US2018/271837,2018,A1 Location in patent:Paragraph 0334-0335