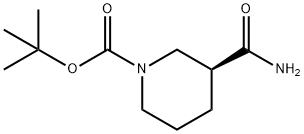
1-Boc-3-carbamoyl piperidine synthesis
- Product Name:1-Boc-3-carbamoyl piperidine
- CAS Number:88466-77-7
- Molecular formula:C11H20N2O3
- Molecular Weight:228.29
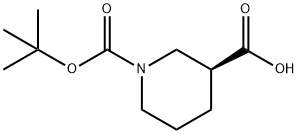
88495-54-9
257 suppliers
$10.00/1g

88466-77-7
25 suppliers
$37.00/250mg
Yield:88466-77-7 100%
Reaction Conditions:
Stage #1: (3S)-1-(tert-butoxycarbonyl)piperidine-3-carboxylic acidwith chloroformic acid ethyl ester;triethylamine in chloroform at 0; for 0.166667 h;
Stage #2: with ammonia in chloroform at 20; for 4 h;
Steps:
10(A); 27(A)
Triethylamine (1.2ImL, 8.72 mmol) and then ethyl chloroformate (0.8 mL, 8.30 mmol) were added dropwise at 00C to a solution of (S)-l-Boc-piperidine-3- carboxylic acid (2 g, 8.72 mmol) in chloroform (40 mL), under nitrogen atmosphere. After stirring 10 min at 0°C, NH3 (gas) was bubbled into the solution for Ih. The reaction mixture was then stirred at room temperature for 3h, 5% NaHCO3 (aq) was added and the phases were separated. The organic layer was dried over sodium sulphate and evaporated under reduced pressure to afford the title compound, which was used for the next step without further purification.Yield: quantitative; LCMS (RT): 3.31 min (Method A); MS (ES+) gave m/z: 229.0 (MH+).
References:
WO2006/123257,2006,A2 Location in patent:Page/Page column 37; 50