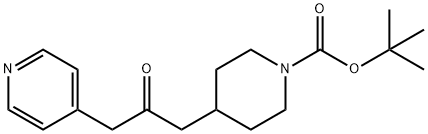
1-BOC-4-(2-OXO-3-PYRIDIN-4-YL-PROPYL)-PIPERIDINE synthesis
- Product Name:1-BOC-4-(2-OXO-3-PYRIDIN-4-YL-PROPYL)-PIPERIDINE
- CAS Number:271577-10-7
- Molecular formula:C18H26N2O3
- Molecular Weight:318.41
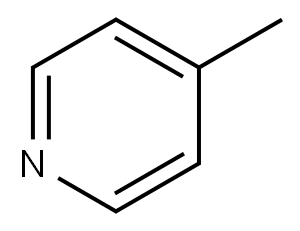
108-89-4
332 suppliers
$14.00/25mL
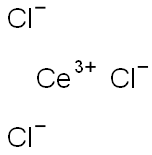
7790-86-5
297 suppliers
$5.00/10g
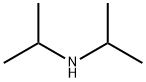
108-18-9
374 suppliers
$10.00/1g

135716-09-5
114 suppliers
$9.00/1g

271577-10-7
11 suppliers
inquiry
Yield:271577-10-7 25%
Reaction Conditions:
with n-butyllithium;lithium diisopropyl amide in tetrahydrofuran;hexane;
Steps:
A.315.2 Step 2
Step 2 Preparation of 1,1-dimethylethyl 4-[2-oxo-3-(4-pyridinyl)propyl]-1-piperidinecarboxylate To a solution of diisopropylamide (6.15 mL, 43.91 mmol) in dry tetrahydrofuran (40 mL) at 0° C. was added 2.5 M butyl lithium solution in hexane (16.22 mL, 40.53 mmol) dropwise over 10 minutes. After the addition, the lithium diisopropylamide solution was stirred at 0° C. for 20 minutes, then cooled to -78° C. 4-Picoline (3.98 mL, 40.53 mmol) was added to the above lithium diisopropylamide solution under N2 dropwise over 10 minutes. The resulting solution was stirred at -78° C. under N2 for 1.5 hours, then transferred into a suspension of anhydrous cerium chloride (10.0 g, 40.53 mmol) in tetrahydrofuran (40 mL) at -78° C. under N2. The mixture was stirred at -78° C. under N2 for 2 hours, then a solution of ethyl 1-[(1,1-dimethylethoxy)carbonyl]-4-piperidineacetate (from step 1 of this Example) (10.98 g, 40.53 mmol) in tetrahydrofuran (40 mL) was added slowly for 1 hour. The mixture was stirred under N2 from -78° C. to room temperature overnight. The reaction was quenched with water, diluted with ethyl acetate, and washed with a pH 7 buffer. The organic layer was washed with water and brine. After filtration, the solvent was removed under reduced pressure to give a crude product mixture. The desired product 1,1-dimethylethyl 4-[2-oxo-3-(4-pyridinyl)propyl]-1-piperidinecarboxylate (3.19 g, 25%) was isolated by chromatography (silica gel, 50:50-75:25-100:0 ethyl acetate/hexane).
References:
US6423713,2002,B1