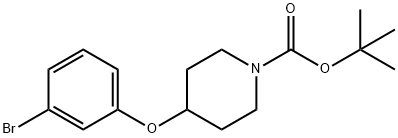
1-BOC-4-(3-bromophenoxy)piperidine synthesis
- Product Name:1-BOC-4-(3-bromophenoxy)piperidine
- CAS Number:790667-54-8
- Molecular formula:C16H22BrNO3
- Molecular Weight:356.25

591-20-8
344 suppliers
$10.00/1g
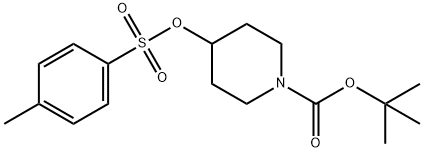
118811-07-7
119 suppliers
$9.00/250mg

790667-54-8
35 suppliers
$163.19/1g
Yield:790667-54-8 86%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 80; for 16 h;
Steps:
50 5.1.50. tert-Butyl 4-(3-bromophenoxy)piperidine-1-carboxylate (27a)
A mixture of 3-bromophenol (25a, 8.00 g, 46.2 mmol), tert-butyl 4-{[(4-methylphenyl)sulfonyl]oxy}piperidine-1-carboxylate (26, 23.0 g, 64.7 mmol), and potassium carbonate (13.8 g, 100 mmol) in DMF (200 mL) was stirred at 80 °C for 16 h. The mixture was partitioned between with AcOEt and water. The organic layer was washed with brine, dried over MgSO4, and concentrated. The residue was purified by silica gel column chromatography (NH, hexane/AcOEt = 100/0 to 90/10) to give the title compound as an oil (14.2 g, 86%). 1H NMR (300 MHz, CDCl3) δ 1.47 (9H, s), 1.64-1.81 (2H, m), 1.84-1.99 (2H, m), 3.27-3.41 (2H, m), 3.62-3.75 (2H, m), 4.39-4.50 (1H, m), 6.79-6.88 (1H, m), 7.03-7.20 (3H, m).
References:
Sato, Kenjiro;Sugimoto, Hiromichi;Rikimaru, Kentaro;Imoto, Hiroshi;Kamaura, Masahiro;Negoro, Nobuyuki;Tsujihata, Yoshiyuki;Miyashita, Hirohisa;Odani, Tomoyuki;Murata, Toshiki [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 5,p. 1649 - 1666]

591-20-8
344 suppliers
$10.00/1g
141699-59-4
235 suppliers
$6.00/1g

790667-54-8
35 suppliers
$163.19/1g

591-20-8
344 suppliers
$10.00/1g

109384-19-2
474 suppliers
$5.00/1g

790667-54-8
35 suppliers
$163.19/1g