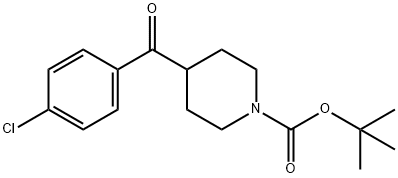
1-BOC-4-(4-CHLORO-BENZOYL)-PIPERIDINE synthesis
- Product Name:1-BOC-4-(4-CHLORO-BENZOYL)-PIPERIDINE
- CAS Number:209808-06-0
- Molecular formula:C17H22ClNO3
- Molecular Weight:323.81

637-87-6
287 suppliers
$38.00/10g
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
183 suppliers
$6.00/100mg

209808-06-0
24 suppliers
inquiry
Yield:209808-06-0 3340 mg
Reaction Conditions:
Stage #1: 1-Chloro-4-iodobenzenewith isopropylmagnesium chloride in tetrahydrofuran; for 2 h;
Stage #2: tert-butyl 4-(methoxy(methyl)carbamoyl)piperidine-1-carboxylate in tetrahydrofuran; for 3 h;
Steps:
20.1-a Step 1-a: Preparation of ferf -butyl 4-(4-chlorobenzoyl)piperidine-l-carboxylate:
To a stirred isopropylmagnesium chloride solution, 2N in THF (8.25 mL; 16.5 mmol) was added a solution of l-chloro-4-iodobenzene (3580 mg; 14.9 mmol) in THF (20 mL) over 5 min. After stirring for 2 h, a solution of tert-butyl 4-[methoxy(methyl)carbamoyl]piperidine-l-carboxylate (4540 mg; 16.3 mmol) in THF (15 mL) was added and the reaction mixture was further stirred for 3 h. Volatiles were removed under reduced pressure and the residue was dissolved in EA (100 mL). The solution was successively washed with a saturated aqueous solution of NH4C1 (3 x 30 mL) and brine (2 x 30 mL), dried over Na2S04, filtered and concentrated to dryness. The residue was purified by column (0865) chromatography (silica gel; PE : EA; 20: 1 to 10: 1 ; v/v) to afford tert-butyl 4-(4-chlorobenzoyl)piperidine- 1 -carboxylate (3340 mg) as a white solid. (0866) MS m/z (+ESI): 324.1, 326.1 [M+H]+. (0867) 'H-NMR (400 MHz, DMSO-i) δ ppm: 8.00 (d, J = 8.8 Hz, 2H), 7.60 (d, J = 8.8 Hz, 2H), 3.98 - 3.94 (m, 2H), 3.65 - 3.55 (m, 1H), 2.95 - 2.82 (m., 2H), 1.77 - 1.73 (m, 2H), 1.46-1.36 (m, 11H).
References:
WO2019/72978,2019,A1 Location in patent:Page/Page column 88

24424-99-5
823 suppliers
$13.50/25G
55695-51-7
100 suppliers
$9.00/250mg

209808-06-0
24 suppliers
inquiry
![1-Boc-4-[(4-chlorophenyl)hydroxyMethyl]piperidine](/CAS/GIF/639468-65-8.gif)
639468-65-8
22 suppliers
inquiry

209808-06-0
24 suppliers
inquiry
1046478-89-0
34 suppliers
$155.00/50mg
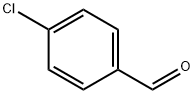
104-88-1
614 suppliers
$5.00/25g

209808-06-0
24 suppliers
inquiry
![1-Piperidinecarboxylic acid, 4-[2-[(4-methoxyphenyl)sulfonyl]hydrazinylidene]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/1510865-53-8.gif)