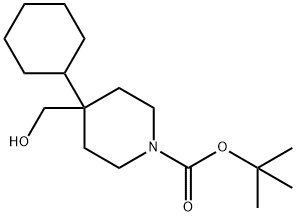
1-Boc-4-cyclohexyl-4-piperidineMethanol synthesis
- Product Name:1-Boc-4-cyclohexyl-4-piperidineMethanol
- CAS Number:312638-87-2
- Molecular formula:C17H31NO3
- Molecular Weight:297.43

24424-99-5
824 suppliers
$13.50/25G
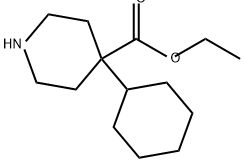
10462-00-7
0 suppliers
inquiry

312638-87-2
12 suppliers
$355.00/250mg
Yield:312638-87-2 84%
Reaction Conditions:
Stage #1: 4-cyclohexylpiperidine-4-carboxylic acid ethyl esterwith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 90 h;
Stage #2: di-tert-butyl dicarbonate in methanol;triethylamine at 20; for 4 h;
Stage #3: with sodium tetrahydroborate in methanol at 0; for 0.5 h;
Steps:
1 1-Boc-4-cyclohexyl-4-piperidinemethanol
To a stirred suspension of lithium aluminium hydride (3 g, 80 mmol) in THF (100 mL) at 0° C. was added a solution of ethyl 4-cyclohexyl-4-piperidine-carboxylate (4.8 g, 20 mmol) in THF (200 mL). The reaction mixture was allowed to warm to rt, stirred for a further 90 hours, then recooled to 0° C. and quenched with saturated aqueous NH4Cl. The resultant grey emulsion was diluted with 3:10:87 Et3N/MeOHEtOAc (500 mL) and filtered through a short pad of celite, rinsing with EtOAc (250 mL). The filtrate was washed with brine, dried over Na2SO4 and evaporated in vacuo to afford a white solid. NMR analysis of the crude product showed it to be a mixture of aldehyde and alcohol (1.5:1). The crude product was dissolved in 1:9 Et3N/MeOH (150 mL). Di-tert-butyldicarbonate (6.5 g, 30 mmol) was added and the solution stirred at rt for 4 hr. Volatiles were removed in vacuo, the residue was dissolved in EtOAc (200 mL), washed successively with saturated aqueous NaHCO3, water and brine, dried over Na2SO4 and concentrated to afford a clear colourless oil. Crude chromatography through silica eluting with 1:1 EtOAc/hexane afforded a mixture of BOC-protected aldehyde and alcohol (1.5:1) as a clear colourless oil. To a solution of this mixture in MeOH (200 mL) at 0° C. was added NaBH4 (378 mg, 10 mmol). The reaction was stirred at 0° C. for 30 min. Solvent was removed in vacuo and the residue partitioned between saturated aqueous NH4Cl (200 mL) and EtOAc (200 mL). The aqueous phase was extracted with EtOAc (200 mL). The combined organic phases were washed with water and brine, dried over Na2SO4 and evaporated to afford the title alcohol as a white solid (4.97 g, 16.7 mmol, 84%). ESI-MS calcd for C17H31NO3: 297; found 297 (M).
References:
US6350760,2002,B1 Location in patent:Page column 48-49

24424-99-5
824 suppliers
$13.50/25G
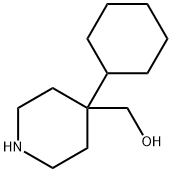
624732-70-3
0 suppliers
inquiry

312638-87-2
12 suppliers
$355.00/250mg
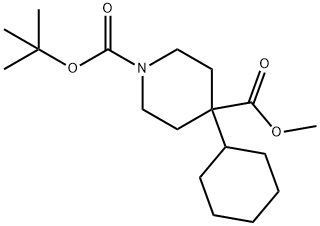
852323-82-1
0 suppliers
inquiry

312638-87-2
12 suppliers
$355.00/250mg

24424-99-5
824 suppliers
$13.50/25G

10462-00-7
0 suppliers
inquiry
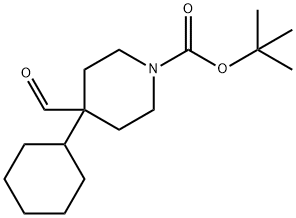
363192-22-7
1 suppliers
inquiry

312638-87-2
12 suppliers
$355.00/250mg
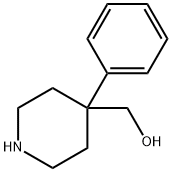
4220-08-0
17 suppliers
$90.00/10mg

312638-87-2
12 suppliers
$355.00/250mg