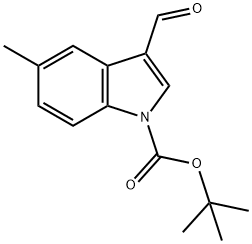
1-Boc-5-methyl-3-formylindole synthesis
- Product Name:1-Boc-5-methyl-3-formylindole
- CAS Number:914348-94-0
- Molecular formula:C15H17NO3
- Molecular Weight:259.3

52562-50-2
138 suppliers
$15.00/250mg

24424-99-5
839 suppliers
$13.50/25G

914348-94-0
41 suppliers
$45.00/50mg
Yield:914348-94-0 0.71 g
Reaction Conditions:
with dmap in acetonitrile at 20;
Steps:
129.1 General procedure F:
General procedure: To a solution of an appropriate indole, azaindole or alternative heterocycles (1.0 eq) and di-ferf-butyl dicarbonate (1eq. to 2 eq., more in particular 1.2 eq) in acetonitrile was added DMAP (0.1 to 0.5 eq. more in particular 0.1 eq). The reaction mixture was stirred overnight at room temperature. The reaction mixture was concentrated under reduced pressure. The residue was dissolved in dichloromethane and washed with a saturated sodium bicarbonate solution. The phases were separated. The aqueous phase was extracted with dichloromethane. The organic phases were combined, washed with a saturated ammonium chloride solution, water and brine, dried over magnesium sulfate, filtered and concentrated under reduced pressure. The BOC-protected compound was used in the next step without further purification.
References:
WO2013/45516,2013,A1 Location in patent:Page/Page column 129; 182
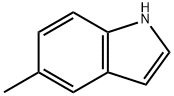
614-96-0
317 suppliers
$19.00/1g

914348-94-0
41 suppliers
$45.00/50mg