
1-BROMO-1,1,2,2-TETRAFLUORO-4-IODOBUTANE synthesis
- Product Name:1-BROMO-1,1,2,2-TETRAFLUORO-4-IODOBUTANE
- CAS Number:129587-49-1
- Molecular formula:C4H4BrF4I
- Molecular Weight:334.88

129587-49-1
18 suppliers
$45.00/50mg
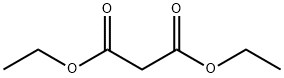
105-53-3
737 suppliers
$5.00/25g
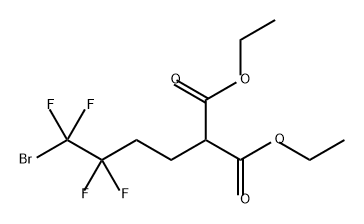
1091592-81-2
0 suppliers
inquiry
Yield:1091592-81-2 74 %
Reaction Conditions:
Stage #1: diethyl malonatewith sodium hydride in N,N-dimethyl-formamide;mineral oil;Inert atmosphere;Cooling with ice;Large scale;
Stage #2: 1-bromo-4-iodo-1,1,2,2-tetrafluoro-butane in N,N-dimethyl-formamide;mineral oil at 100;Inert atmosphere;Large scale;
Steps:
1.1 (First step) Production of 2-(4-bromo-3,3,4,4-tetrafluorobutyl)-malonic acid diethyl ester
Under a nitrogen atmosphere, 320 g of sodium hydride (containing 60% mineral oil) (7.17 mol) was added to 1900 mL of dimethylformamide, and 1208 g (7.17 mol) of diethyl malonate was added under an ice bath.After stirring for 1 hour, 2400 g (7.17 mol) of 1-bromo-4-iodo-1,1,2,2-tetrafluoro-butane was added to this solution while controlling the temperature of the reaction solution to 100° C. or lower.After stirring for 1 hour, 1000 mL of 1N hydrochloric acid aqueous solution was added, and the mixture was extracted with diisopropyl ether.The organic layer was concentrated and distilled under reduced pressure (115-116° C./0.53 kPa) to obtain 1940 g of the target 2-(4-bromo-3,3,4,4-tetrafluorobutyl)-malonic acid diethyl ester ( Yield 74%, purity 89 GC area %) was obtained as a pale yellow liquid.
References:
JP2022/51549,2022,A Location in patent:Paragraph 0098-0100