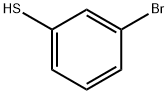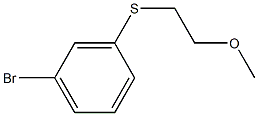
1-bromo-3-(2-methoxyethylsulfanyl)benzene synthesis
- Product Name:1-bromo-3-(2-methoxyethylsulfanyl)benzene
- CAS Number:1021169-59-4
- Molecular formula:C9H11BrOS
- Molecular Weight:247.152
Yield:1021169-59-4 81%
Reaction Conditions:
Stage #1: 3-Bromothiophenolwith sodium hydride in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: 2-Bromoethyl methyl ether in N,N-dimethyl-formamide; for 17 h;
Steps:
1.17.A
Step A: Preparation of Intermediate (3-Bromophenyl)(2-methoxyethyl)sulfane.To a suspension of sodium hydride (96.6 mg, 2.42 mmol) in DMF (4.2 mL) was added 3-bromobenzenethiol (0.22 mL, 2.132 mmol). After stirring at room temperature for 30 min, 1- bromo-2-methoxyethane (0.22 mL, 2.341 mmol) was added to the reaction mixture. After stirring for 17 h, the reaction mixture was quenched with H2O and extracted with EtOAc. The combined organic layers were washed with H2O, brine, dried over MgSO4 and concentrated. The residue was purified through a silica gel column with 15% EtOAc/ 85% hexanes to afford the title compound (425.1 mg, 81%) as a colorless oil. 1H NMR (400 MHz, Methanol-^) δ ppm 3.13 (t, J= 6.5 Hz, 2 H), 3.33 (s, 3 H), 3.57 (t, J = 6.5 Hz, 2 H), 7.20 (dd, J= 7.9, 7.9 Hz, 1 H), 7.30 - 7.35 (m, 2 H), 7.50 - 7.52 (m, 1 H).
References:
WO2008/48609,2008,A1 Location in patent:Page/Page column 64