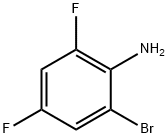
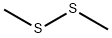1-Bromo-3,5-difluoro-2-methylsulfanylbenzene synthesis
- Product Name:1-Bromo-3,5-difluoro-2-methylsulfanylbenzene
- CAS Number:861931-33-1
- Molecular formula:C7H5BrF2S
- Molecular Weight:239.08
Yield:861931-33-1 66%
Reaction Conditions:
with isopentyl nitrite at 75 - 95; for 1 h;
Steps:
12
Dissolve 2-bromo-4,6-difluoroaniline (40 g, 192 mmol) in methyldisulfide (250 mL) and heat to 75 °C under nitrogen. Add isoamyl nitrite (67 mL, 500 mmol) dropwise via an addition funnel trough a reflux condenser (-1 drop/sec). Large exotherm may occur if addition is too fast. After addition is complete, heat the reaction to 95 °C for 1 hour and cool to room temperature and concentrate in vacuo. Purify dark brown residue via silica gel chromatography eluting with hexanes to yield 30.3 g of 1-bromo-3, 5- difluoro-2-methylsulfanyl-benzene (66%). Charge a flask with isopropyl magnesium chloride (145 mL, 2M in THF, 290 mmol) and dilute with tetrahydrofuran (150 mL) and heat to 40 °C under nitrogen. Add 1-bromo-3, 5-difluoro-2-methylsulfanyl-benzene (28 g, 117 mmol) slowly over 5 minutes. After 30 minutes, cool the reaction to 0 °C and add trimethylborate (46 mL, 410 mmol) diluted with tetrahydrofuran (100 mL) via an addition funnel over 5 minutes. Partition the resulting gelatinous mixture between methylene chloride and 1N HC1. Acidify the aqueous layer to pH-1 (if needed) and vigorously stir the biphasic mixture until all solids are dissolved. the organic layer. Dry over sodium sulfate, filter and concentrate. Triturate with hexanes and filter to yield 14.7 g (61 %) of the title compound.
References:
WO2005/73204,2005,A1 Location in patent:Page/Page column 79