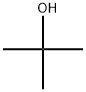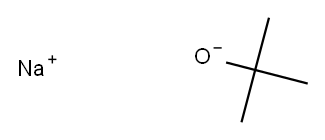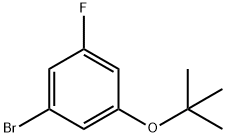
1-BroMo-3-(tert-butoxy)-5-fluorobenzene synthesis
- Product Name:1-BroMo-3-(tert-butoxy)-5-fluorobenzene
- CAS Number:889362-81-6
- Molecular formula:C10H12BrFO
- Molecular Weight:247.1
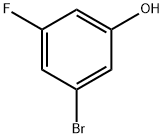
433939-27-6
184 suppliers
$5.00/1g

24424-99-5
820 suppliers
$13.50/25G

889362-81-6
15 suppliers
inquiry
Yield:889362-81-6 68%
Reaction Conditions:
with anhydrous magnesium perchlorate at 50 - 65; for 6.33333 h;Inert atmosphere;
Steps:
1.1 (Step 1) Preparation of 1-bromo-3-(tert-butoxy)-5-fluorobenzene
To 3-bromo-5-fluorophenol (500 mg) were sequentially added di-tert-butyl dicarbonate (1.14 g) and magnesium perchlorate (58 mg) at room temperature under argon flow. The reaction mixture was stirred at 50° C. for 1 hour 20 minutes. To the reaction mixture was added at 50° C. di-tert-butyl dicarbonate. The reaction mixture was stirred at 50° C. for 1 hour and further stirred at 65° C. for 1 hour, and then cooled to room temperature. To the reaction mixture was added at room temperature di-tert-butyl dicarbonate. The reaction mixture was stirred at 65° C. for 3 hours. The reaction mixture was cooled to room temperature, and thereto was added a mixed solution of n-hexane/ ethyl acetate (1/1). The reaction mixture was sequentially washed with 3N hydrochloric acid, saturated aqueous sodium hydrogen carbonate solution, and brine, and then dried over sodium sulfate and concentrated. The residue was purified by silica gel column chromatography (eluent: n-hexane/ethyl acetate=1/0 to 20/1) to give the title compound (437 mg) in the yield of 68%. (0174) 1H-NMR (CDCl3) δ: 1.35 (s, 9H), 6.62-6.66 (m, 1H), 6.92-6.98 (m, 2H).
References:
US2019/352284,2019,A1 Location in patent:Paragraph 0172-0174