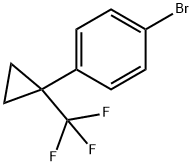
1-Bromo-4-(1-trifluoromethyl-cyclopropyl)-benzene synthesis
- Product Name:1-Bromo-4-(1-trifluoromethyl-cyclopropyl)-benzene
- CAS Number:1227160-18-0
- Molecular formula:C10H8BrF3
- Molecular Weight:265.07

883547-73-7
31 suppliers
$74.00/1g

1227160-18-0
46 suppliers
$55.00/250mg
Yield: 100%
Reaction Conditions:
with zinc bromide on montmorillonite in N,N-dimethyl-formamide at 20;Inert atmosphere;Darkness;
Steps:
121A.1 Step 1:
1.40 g (6.22 mmol) of zinc bromide were initially introduced into 56 ml of methanol, 5.64 g of montmorillonite K10 were added and the mixture was stirred at RT for 1 h.
After removal of the methanol, the powder which remained was heated in a sand bath at a bath temperature of 200° C. for 1 h and then allowed to cool under argon.
The title compound was then prepared as follows:
10.0 g (53.7 mmol) of 1-phenyl-1-(trifluoromethyl)cyclopropane were initially introduced into 50 ml of pentane. 6.1 g (5.37 mmol) of the activated zinc bromide on montmorillonite obtained above were added and 27.7 ml (537 mmol) of bromine were then slowly added dropwise in the dark, while stirring.
The mixture was then stirred further at RT in the dark overnight. 150 ml of a saturated aqueous sodium sulfite solution were subsequently slowly added dropwise, while cooling with ice, and the mixture was stirred at RT for a further approx.
30 min until it was decolorized.
The solid was filtered off and rinsed twice with pentane.
After separation of the filtrate phases, the aqueous phase was extracted twice with 200 ml of pentane each time.
The combined organic phases were dried over sodium sulfate, filtered and concentrated under gentle conditions (significant volatility of the target compound).
17.1 g (>100% of th.) of the title compound were obtained in this manner, which according to 1H-NMR still contained pentane.
1H-NMR (400 MHz, CDCl3, δ/ppm): 7.47 (d, 2H), 7.32 (s, 2H), 1.39-1.30 (m, 2H), 1.04-0.95 (m, 2H).
GC/MS (method L, ESIpos): Rt=3.45 min, m/z=264/266 [M+H]+.
References:
BAYER SCHERING PHARMA AKTIENGESELLSCHAFT;Härter, Michael;Beck, Hartmut;Ellinghaus, Peter;Berhörster, Kerstin;Greschat, Susanne;Thierauch, Karl-Heinz;Süssmeier, Frank US2013/196964, 2013, A1 Location in patent:Paragraph 1340; 1341; 1342; 1343; 1344
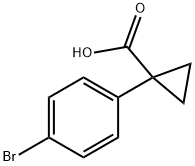
345965-52-8
160 suppliers
$7.00/250mg

1227160-18-0
46 suppliers
$55.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1227160-18-0
46 suppliers
$55.00/250mg
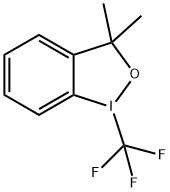
887144-97-0
161 suppliers
$28.00/250mg

345965-52-8
160 suppliers
$7.00/250mg

1227160-18-0
46 suppliers
$55.00/250mg
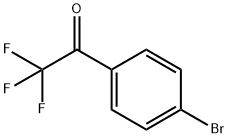
16184-89-7
170 suppliers
$6.00/1g

1227160-18-0
46 suppliers
$55.00/250mg