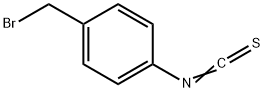
1-BROMO-4-ISOTHIOCYANATOMETHYLBENZENE synthesis
- Product Name:1-BROMO-4-ISOTHIOCYANATOMETHYLBENZENE
- CAS Number:155863-32-4
- Molecular formula:C8H6BrNS
- Molecular Weight:228.11
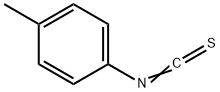
622-59-3
109 suppliers
$27.00/1g

155863-32-4
25 suppliers
inquiry
Yield:155863-32-4 48%
Reaction Conditions:
with N-Bromosuccinimide;dibenzoyl peroxide in tetrachloromethane; for 8 h;Reflux;
Steps:
4.1.3. Synthesis of 1-(bromomethyl)-4-isothiocyanatobenzene (5)
Compound 5 was synthesized as described previously [20]. First, 2.6 g (17.5 mmol) of p-Tolylisothiocyanate was dissolved in 40 mL of CCl4 in a round-bottom flask. Then, 3.2 g (18 mmol) N-bromosuccinimide was added, followed by a small amount of benzoyl peroxide. The mixture was stirred at reflux for 8 h and then filtered when cooled down to room temperature. The solvent was evaporated, and the crude product was recrystallized in methanol. The product was obtained as pale yellow needle-like crystals (1.9 g (48%)). 1H NMR (400 MHz, DMSO-d6) δ 7.52 (d, J = 8.6 Hz, 2H), 7.42 (d, J = 8.6 Hz, 2H), 4.72 (s, 2H). 13C NMR (100 MHz, acetone-d6) 141.1, 141.1, 139.0, 131.6, 126.9, 33.2.
References:
Baun, Christina;Jensen, Andreas Ingemann;Nielsen, Karin Michaelsen;Pedersen, Kristina S?borg;Thisgaard, Helge;Zhuravlev, Fedor [Molecules,2020,vol. 25,# 5]