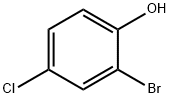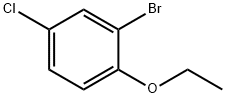
1-Bromo-5-chloro-2-ethoxybenzene synthesis
- Product Name:1-Bromo-5-chloro-2-ethoxybenzene
- CAS Number:1225577-71-8
- Molecular formula:C8H8BrClO
- Molecular Weight:235.51
Yield:1225577-71-8 98%
Reaction Conditions:
with caesium carbonate at 20; for 2 h;
Steps:
20
2-bromo-4-chloro-l-ethoxybenzeneTo a solution of 2-Bromo-4-chlorophenol (2.12 g, 10.2 mmol) in 25 niL acetone was added iodoethane (0.850 mL, 10.6 mmol) and cesium carbonate (4.08 g, 12.5 mmol). The reaction mixture was stirred at 500C for 2 hours. The reaction mixture was partitioned between ethyl acetate and water, and the organic portion washed with brine, dried over magnesium sulfate, and concentrated to yield 2.37 g (98%) of 2-bromo-4-chloro-l-ethoxybenzene as a yellow oil, which was carried forward without further purficiation. LCMS (ESI) no m/z signal; 1H NMR (400MHz, DMSO-J6) δ: 7.67 (d, J = 2.6, IH), 7.39 (dd, / = 8.8, 2.6, IH), 7.12 (d, / = 8.9, IH), 4.11 (q, J= 7.0, 2H), 1.35 (t, J= 7.0, 3H).
References:
WO2011/3065,2011,A2 Location in patent:Page/Page column 101