
1-BROMO-5-DIFLUOROMETHYL-2-FLUOROBENZENE synthesis
- Product Name:1-BROMO-5-DIFLUOROMETHYL-2-FLUOROBENZENE
- CAS Number:886509-99-5
- Molecular formula:C7H4BrF3
- Molecular Weight:225.01

886509-99-5
32 suppliers
$49.00/1g

73183-34-3
568 suppliers
$6.00/5g
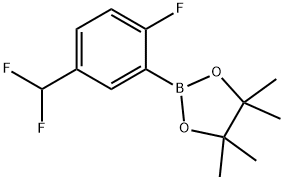
1142228-23-6
20 suppliers
inquiry
Yield:1142228-23-6 65%
Reaction Conditions:
with anhydrous potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II) dichloromethane adduct in 1,4-dioxane at 90; for 18 h;Inert atmosphere;
Steps:
T26
2-(5-(Difluoromethyl)-2-fluorophenyl)-4,4,5,5-tetramethyl-1,3,2- dioxaborolane (T26.1). A stirred mixture of l-bromo-5-difluoromethyl-2-fluorobenzene (commercially available from Oakwood Products, Inc.) (2.0231 g, 8.991 mmol), bis(pinacolato)diboron (2.5123 g, 9.893 mmol), dichloro[l,l'- bis(diphenylphosphino)ferrocene]palladium (II) DCM adduct (0.3688 g, 0.4516 mmol), and potassium acetate (2.6504 g, 27.01 mmol) in dry 1,4-dioxane (35 mL) was purged with argon and placed under vacuum and the purging vacuum process repeated three times. The mixture was heated to 90°C and monitored with LC-MS and TLC. After 18 hours, the reaction was cooled to room temperature and then filtered through C6lite filter aid. The organic solvent was removed under reduced pressure, and the residue was purified on a 40 g column of silica gel (0-10% EtOAc in hexanes) to afford T26.1 as a colorless oil that was used without further purification (1.6019 g, 65% yield). 1H NMR (400 MHz, CDCl3) δ ppm 7.89 (1 H, td, J=2.7, 1.2 Hz), 7.63 (1 H, m), 7.09 (1 H, t, J=8.6 Hz), 6.62 (1H, t), 1.35 (12 H, s).
References:
WO2009/111056,2009,A1 Location in patent:Page/Page column 233