
1-Bromo-8-chloronaphthalene synthesis
- Product Name:1-Bromo-8-chloronaphthalene
- CAS Number:20816-79-9
- Molecular formula:C10H6BrCl
- Molecular Weight:241.51
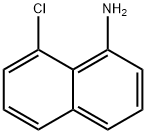
59107-51-6
92 suppliers
inquiry

20816-79-9
137 suppliers
inquiry
Yield:20816-79-9 72%
Reaction Conditions:
with toluene-4-sulfonic acid;copper(I) bromide;sodium nitrite in acetonitrile at -5 - 25; for 12 h;
Steps:
C 1 -bromo-8-chloro-naphthalene
To a solution of 8-chloronaphthalen-l -amine (57 g, 320 mmol, 1 eq) and TsOH*H20 (219 g, 1.16 mol, 3.6 eq) in MeCN (1000 mL) was added a solution ofNaN02 (39.8 g, 577 mmol, 1.8 eq) and CuBr (138 g, 963 mmol, 29.3 mL, 3 eq) in H20 (120 ml,) at - 5 °C, then the reaction mixture was stirred at 25 °C for 12 hours. The reaction mixture was added saturated Na2S03 solution (100 mL) and stirred for 15 mins, then extracted with ethyl acetate (1000 mL><3). The combined organic layers were washed with brine (500 mL), dried over Na2S04, filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (Si02, Petroleum ether). Title compound 1- bromo-8-chloro-naphthalene (56 g, 229 mmol, 72% yield, 99% purity) was obtained as white solid. (0511) [0313] NMR (400MHz, chloroform-d) 5 = 7.93 (dd, J= 1.2, 7.6 Hz, 1H), 7.82 (dd, J= 1.2, 8.4, 1H), 7.79 (dd, ./= 1.2, 8.4, HI), 7.67 (dd, ./ = 1.2, 7.6 Hz, 1H), 7.37 (t, J= 8.0 Hz, 1 H), 7.28 (t, .7= 8.0 Hz, III).
References:
WO2020/47192,2020,A1 Location in patent:Paragraph 0309; 0312-0313
![1H-NAPHTHO[1,8-DE][1,2,3]TRIAZINE](/CAS/GIF/204-03-5.gif)
204-03-5
7 suppliers
inquiry

20816-79-9
137 suppliers
inquiry
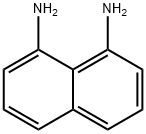
479-27-6
363 suppliers
$6.00/10g

20816-79-9
137 suppliers
inquiry