
1-BROMOMETHYL-3-METHYL-2-NITRO-BENZENE synthesis
- Product Name:1-BROMOMETHYL-3-METHYL-2-NITRO-BENZENE
- CAS Number:55324-02-2
- Molecular formula:C8H8BrNO2
- Molecular Weight:230.06
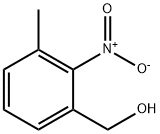
80866-76-8
88 suppliers
$35.00/1g

55324-02-2
28 suppliers
$85.00/1g
Yield:55324-02-2 100%
Reaction Conditions:
with dibromotriphenylphosphorane in acetonitrile at 0 - 20;
Steps:
22 Preparation 22; 3-METHYL-2-NITROBENZYL BROMIDE
A round bottomed flask was charged with 5.0 G (0. 029 mol) of 3- METHYL-2-NITROBENZYL ALCOHOL and 100 mL of anhydrous acetonitrile. The reaction solution was cooled to 0 °C and treated, portionwise, with 13.2 G (0.031 mol) TRIPHENYLPHOSPHINE perbromide (PH3P-BR2). The reaction was allowed to warm to rt overnight at which point the solvent was removed and the resulting solids triturated with 50: 50 ether: hexanes. The wash solvent was filtered through a fine frit and then concentrated to give a quantitative yield (7.03 G) of the desired material as a white SOLID. 1H NMR (400 MHz, CDCI3) S 7.36-7. 34 (m, 2H), 7.33-7. 24 (m, 1H), 4.45 (s, 2H), 2.33 (s, 3H).
References:
WO2004/41793,2004,A1 Location in patent:Page 63-64
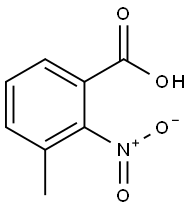
5437-38-7
445 suppliers
$5.00/10g

55324-02-2
28 suppliers
$85.00/1g
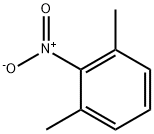
81-20-9
8 suppliers
$17.00/25g

55324-02-2
28 suppliers
$85.00/1g