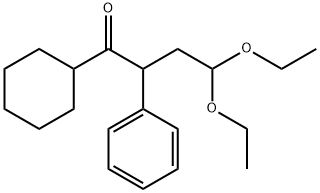
1-Butanone, 1-cyclohexyl-4,4-diethoxy-2-phenyl- synthesis
- Product Name:1-Butanone, 1-cyclohexyl-4,4-diethoxy-2-phenyl-
- CAS Number:1347677-19-3
- Molecular formula:C20H30O3
- Molecular Weight:318.45
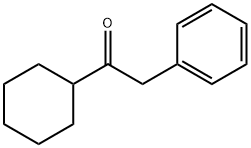
61259-29-8
29 suppliers
$23.00/1g
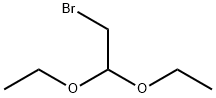
2032-35-1
479 suppliers
$5.00/10g

1347677-19-3
5 suppliers
inquiry
Yield:1347677-19-3 42%
Reaction Conditions:
Stage #1: 1-cyclohexyl-2-phenylethanonewith sodium hydride in DMF (N,N-dimethyl-formamide) at 20; for 1 h;
Stage #2: Bromoacetaldehyde diethyl acetal in DMF (N,N-dimethyl-formamide) at 20 - 80; for 2 h;
Steps:
5 Example 5 (Comparative); 1- [[ (RS, SR)-4-CYCLOHEXYL-4-HYDROXY-3-PHENYL-BUTYL]-4- (2-METHOXYPHENYL)-PIPERAZINE]; 4-Cyclohexyl-4-oxo-3-phenyl-butyraldehyde diethyl acetal (Compound 5b)
To a solution of 1.78 g of Compound 5a in 30 ml of anhydrous dimethylformamide were added at r. t. 0.37 g of 60% sodium hydride oil dispersion and the mixture was stirred at r. t. for 1 h. 1.46 ml of 2-bromoacetaldeide diethyl acetal was added and the mixture stirred at r. t. for 1 h and at [80°C] for 1 h, cooled to r. t. , quenched with water and extracted with EtOAc. The collected organic layers were washed with water, dried [(NA2SO4)] and the solvent was evaporated under vacuum. The crude was purified by flash chromatography eluting with petroleum ether-EtOAc 95: 5 to give 1.17 g (42%) of the title compound. 'H-NMR (200 [MHZ,] [CDC13,] [6)] : 1.01-1. 98 (m, 17H), 2.28-2. 48 [(M,] 2H), 3.30-3. 71 (m, 4H), 3.98 [. (T,] 1H), 4.25 (t, 1H), 7.12-7. 39 [(M,] 5H)
References:
WO2003/106444,2003,A1 Location in patent:Page 32