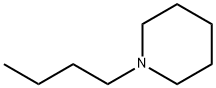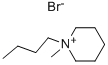
1-Butyl-1-methylpiperidinium Bromide synthesis
- Product Name:1-Butyl-1-methylpiperidinium Bromide
- CAS Number:94280-72-5
- Molecular formula:C10H22BrN
- Molecular Weight:0
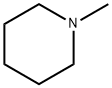
626-67-5
219 suppliers
$15.00/25g
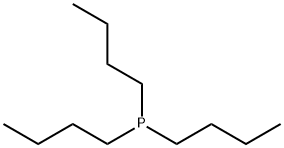
998-40-3
343 suppliers
$18.32/25g
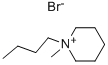
94280-72-5
78 suppliers
$14.00/5g
Yield: 90%
Reaction Conditions:
at 89.84 - 99.84; for 48 h;Inert atmosphere;
Steps:
SI2-1-7. Tributyldecylphosphonium bromide, [P444,10 ]Br.
[P444,10]Br was synthesized as the precursor for the [P444,10]+-based ionic liquids. 1-Bromodecane (TokyoChemical Industry, 7.10 mL, 34.3 mmol) was added to tri-n-butylphosphine (Kanto Chemical, 7.00 mL, 28.4mmol) at a nitrogen atmosphere in a flask equipped with a reflux condenser and a magnetic stirrer. Thesolution was then heated to 363-373 K in an oil bath for 2 days while stirring. The solution was allowed tocool to room temperature and condensed by evaporation. The ionic liquid layer was washed several timeswith n-hexane and dried in vacuo at 313 K for more than 2 days. The yield was 90.0%. 1H NMR (400 MHz,DMSO-d6): δ/ppm = 0.85 (t, J = 6.9 Hz, 3H, PC9H18CH3), 0.91 (t, J = 7.2 Hz, 9H, P(C3H6CH3)3), 1.24 (m,12H, PC3H6C6H12CH3), 1.33-1.49 (m, 16H, C7H15C2H4CH2P(CH2C2H4CH3)3), 2.18-2.26 (m, 8H,C9H19CH2P(CH2C3H7)3).
References:
Shirota, Hideaki;Ando, Masatoshi;Kakinuma, Shohei;Takahashi, Kotaro [Bulletin of the Chemical Society of Japan,2020,vol. 93,# 12,p. 1520 - 1539] Location in patent:supporting information

626-67-5
219 suppliers
$15.00/25g

109-65-9
551 suppliers
$10.00/10g
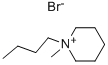
94280-72-5
78 suppliers
$14.00/5g