
1-CBZ-3-BOC-AMINO PYRROLIDINE synthesis
- Product Name:1-CBZ-3-BOC-AMINO PYRROLIDINE
- CAS Number:185057-49-2
- Molecular formula:C17H24N2O4
- Molecular Weight:320.38

99724-19-3
178 suppliers
$10.00/1g

501-53-1
393 suppliers
$10.00/1g

185057-49-2
11 suppliers
inquiry
Yield:185057-49-2 83%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20; for 24 h;
Steps:
1.A; 4.B; 5.B
[0204] A. 1-Cbz-3-(t-butyloxycarbonyl)aminopyrrolidine [CHEMMOL-00014] [0205] To a solution of 3-(t-butyloxycarbonyl)aminopyrrolidine (5.0 g, 26.8 mmol) in THF (40 mL) was added triethylamine (3.7 mL, 26.8 mmol), followed by the addition of benzyl chloroformate (3.8 mL, 26.8 mmol) slowly. After the reaction mixture was stirred for 24 h at room temperature, the reaction mixture was filtered and solvent removed in vacuo. The residue was dissolved in EtOAc and washed sequentially with 1 N NaHCO3 (100 mL), 1.5 N citric acid (100 mL), and water. The organic layer was dried (MgSO4) and concentrated to dryness in vacuo to give the title compound (7.13 g, 83%). [0206] TLC Rf (C) 0.41; [0207] MS 321 (M+H)+; [0208] Analysis for C17H24N2)4: [0209] Calcd: C, 63.73; H, 7.55; N, 8.74; [0210] Found: C, 64.01, H, 7.39, N, 8.71.; [0304] B. 1-Cbz-3-(t-butyloxycarbonyl)aminopyrrolidine [0305] The title compound was prepared according to the procedure described in Example 1A.; 1-Cbz-(t-butyloxycarbonyl)aminopyrrolidine [0287] The title compound may be prepared according to the procedure described in Example 1A.
References:
US2004/10017,2004,A1 Location in patent:Page/Page column 11; 15; 16
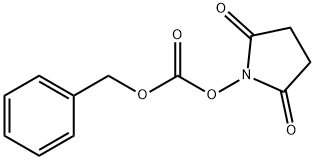
13139-17-8
503 suppliers
$6.00/25g

99724-19-3
178 suppliers
$10.00/1g

185057-49-2
11 suppliers
inquiry

24424-99-5
825 suppliers
$13.50/25G

185057-49-2
11 suppliers
inquiry

24424-99-5
825 suppliers
$13.50/25G
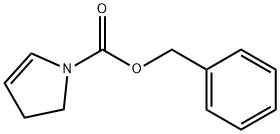
68471-57-8
48 suppliers
$15.00/100mg

185057-49-2
11 suppliers
inquiry
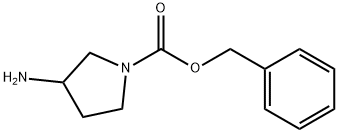
185057-50-5
110 suppliers
$35.00/1g

185057-49-2
11 suppliers
inquiry