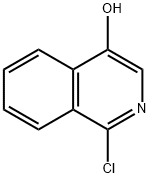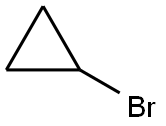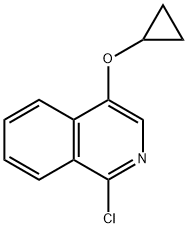
1-chloro-4-(cyclopropyloxy)isoquinoline synthesis
- Product Name:1-chloro-4-(cyclopropyloxy)isoquinoline
- CAS Number:1409964-36-8
- Molecular formula:C12H10ClNO
- Molecular Weight:219.67
Yield:1409964-36-8 32.7%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 145; for 18 h;Sealed tube;
Steps:
1 Preparation of 1-chloro-4-cyclopropoxyisoquinoline
To a solution of 1-chloroisoquinolin-4-ol (1.0 g, 55.7 mmol) in DMF (20 m) was added Cs2CO3(2.72 g, 8.35 mmol) followed by cyclopropylbromide (6.74 g, 55.7 mmol) at room temperature. The reaction vessel (Pressure tube) was sealed and heated at 145° C. for 18 h. The reaction mass was diluted with water and extracted with ethyl acetate. The combined organic layer was dried over anhydrous Na2SO4and evaporated under reduced pressure to get crude compound. The crude compound was purified by silica gel chromatography to get desired compound (0.4 g, 32.7%) as a yellow solid.1H NMR (400 MHz, CDCl3): δ ppm 8.22-8.24 (m, 2H), 8.14 (s, 1H), 8.14-8.12 (m, 1H), 7.74-7.67 (m, 2H), 4.00-3.95 (m, 1H), 0.92-0.90 (m, 4H); MS: MS m/z 220.0 (M++1).
References:
US9527885,2016,B2 Location in patent:Page/Page column 562; 563