
1-chloroethyl propyl carbonate synthesis
- Product Name:1-chloroethyl propyl carbonate
- CAS Number:99464-82-1
- Molecular formula:C6H11ClO3
- Molecular Weight:166.6
Yield:99464-82-1 84%
Reaction Conditions:
with pyridine in diethyl ether at 0 - 20; for 4.83333 h;Inert atmosphere;
Steps:
5 Preparation of propyl-1-chloroethyl carbonate.
A solution of propyl alcohol (70 mmol) and 1-chloroethyl chloroformate (7.6 mL, 70 mmol) in diethyl ether (200 mL) was cooled to 0 ° C. under argon. Pyridine (70 mmol) was added dropwise over 5 minutes with stirring. The solution was stirred at 0 ° C. for 15 minutes, then warmed to room temperature and stirred for an additional 4.5 hours. The ether solution was filtered, washed with 1 M HCl, then washed twice with water, dried over MgSO 4, filtered and concentrated on a rotary evaporator to give propyl-1-chloroethyl carbonate (9.8 g , 84%). Anhydrous PMPA (0.3 g, 1 mmol) and DIEA (0.7 mL, 4 mmol) were placed in anhydrous DMF (2 mL) under argon. Propyl-1-chloroethyl carbonate (3 mmol) was then added and the suspension was stirred at 50 ° C. for 20 hours. DMF was removed with a rotary evaporator. And the reaction was partitioned between CH 2 Cl 2 and water. The CH 2 Cl 2 layer was dried over magnesium sulfate, filtered and concentrated on a rotary evaporator. The residue was taken up in methylene chloride and applied to a silica gel column (25 g, SiO 2). This was eluted with 100 mL of CH 2 Cl 2, 3, 6, 9, 12, 15, 18% (v / v) isopropanol in methylene chloride 50 mL, then 21%, 200 mL of isopropanol in methylene chloride. Appropriate fractions were pooled and evaporated to give the desired product.
References:
JP2015/164934,2015,A Location in patent:Paragraph 0192-0194
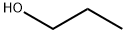
71-23-8
749 suppliers
$12.00/100g
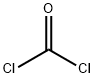
75-44-5
2 suppliers
inquiry

75-07-0
416 suppliers
$14.00/5mL

99464-82-1
4 suppliers
inquiry
