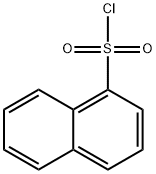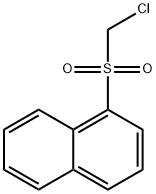
1-[(CHLOROMETHYL)SULFONYL]NAPHTHALENE synthesis
- Product Name:1-[(CHLOROMETHYL)SULFONYL]NAPHTHALENE
- CAS Number:87491-79-0
- Molecular formula:C11H9ClO2S
- Molecular Weight:240.71
Yield:87491-79-0 88.8%
Reaction Conditions:
Stage #1: 1-Naphthalenesulfonyl chloridewith sodium hydrogencarbonate;sodium sulfite in water at 40 - 100; for 1.5 - 1.66667 h;
Stage #2: chlorobromomethanewith tetrabutylammomium bromide in water at 40 - 75; for 14.5 h;Product distribution / selectivity;
Steps:
14.1; 69.2
Step 1 1-Chloromethanesulfonyl-naphthalene A mixture of 1-naphthalene sulfonyl chloride (20.2 g, 89.1 mmol), sodium sulfite (22.5 g, 178 mmol) and sodium bicarbonate (15.1 g, 180 mmol) in water (125 mL) was stirred at 100° C. for one hour. After cooling to ambient temperature for 40 minutes, bromochloromethane (90 mL, 1.4 mol) and tetrabutylammonium bromide (2.87 g, 8.91 mmol) were added. The reaction mixture was then stirred at 75° C. for 14.5 hours. After cooling to ambient temperature, the layers of the reaction mixture were separated, and the organic phase was concentrated. The residue was purified by flash chromatography with 100% ethyl acetate. Hexane was added after concentration to help solidify, and the mixture was again concentrated. Drying at 80° C. in vacuo for 20 minutes yielded 1-chloromethanesulfonyl-naphthalene as a pale yellow solid (19.0 g, 88.8%). MP 103-5° C. Mass Spectrum (+El, M+) m/z 240. 1HNMR (500 MHz, DMSO-d6): δ8.64-5 (m, 1H), 8.41 (d, 1H, J=8.23 Hz), 8.27 (dd, 1H, J=7.33 Hz and 1.22 Hz), 8.16-8.18 (m, 1H), 7.71-7.81 (m, 3H), 5.40 ppm, (s, 2H). Elemental Analysis for C11H9ClO2S: Calcd: C, 54.89; H, 3.77; N, 0.00; Found: C, 54.98; H, 3.81; N, 0.00.; Step 2 1-Chloromethane-sulfonyl-naphthalene A mixture of naphthalene-1-sulfonyl chloride (10.0 g, 44 mmoles), sodium sulfite (11.12 g, 88 mmoles), and sodium bicarbonate (7.4 g, 88 mmoles) in water (50 mL) was heated to 100° C. for one hour. The crude sodium sulfinate solution was allowed to cool for 30 minutes, and then treated with bromochloromethane (43 mL, 661 mmoles) and tetra-N-butylammonium bromide (1.4 g, 4.4 mmoles). The resultant mixture was heated to 75° C. overnight. All solvents were removed under vacuum. Compound was recrystallized from CH2Cl2/hexane to give the title compound as an off white solid (10.62 g, 44 mmoles).
References:
US2007/37802,2007,A1 Location in patent:Page/Page column 23; 61