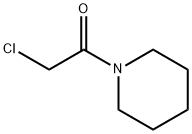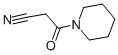
1-CYANOACETYLPIPERIDINE synthesis
- Product Name:1-CYANOACETYLPIPERIDINE
- CAS Number:15029-30-8
- Molecular formula:C8H12N2O
- Molecular Weight:152.19

110-89-4
0 suppliers
$15.96/100ML
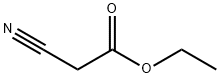
105-56-6
461 suppliers
$10.00/5ml

15029-30-8
81 suppliers
$12.00/1g
Yield: 92%
Reaction Conditions:
in neat (no solvent) at 20;Inert atmosphere;
Steps:
Preparation of N-substituted cyanacetamides 1 and 4 - precursors to the cyanoximes
Neat ethylcyanoacetate reacts at room temperature with neat secondary amines at solvent-free conditions to form respective amides [44,45]. The general procedure involves mixing neat piperidine or morpholine with NC-CH2-C(O)OC2H5 at 1.5:1 ratio under stirring and an N2 blanket. These reactions within 24-60h at room temperature lead to prePiPCO (1) and preMCO (4) in high yields (Scheme 1). Course of both reactions was monitored by TLC watching disappearance of the cyanoacetylacetate. Since starting compounds and final cyan-acetamides do not contain chromophores, an I2-chamber was used for plates’ development. Thus, thick off-white precipitates of 1 and 4 were filtered under vacuum, washed with small portions (∼20mL) of toluene and hexane (∼25mL), and then dried in a vacuum dessicator charged with concentrated H2SO4 for several days until smell of the amine was no longer detectable. Data for 1: yield 92%, m.p. 87-90°C. The ring methylene protons in both precursors and target cyanoximes demonstrate complex non-first order 1H NMR spectra of AA′BB′ spin systems. The 1H NMR, ppm, in dmso-d6: 4.01 (singlet, 2H), ring protons - 3.41 (“triplet”, 2H), 3.28 (“tiplet”, 2H), 1.53 (multiplet, 6H). Mass-spectrometry, positive FAB (Ar): for C8H12N2O, calculated - 152.12 (found - 152.1). Data for 4: yield ∼100%, m.p. 78-81°C, Rf=0.12 in EtOAc/hexane (1:2) mobile phase. The 1H NMR, ppm, in dmso-d6: 4.03 (singet, 2H), ring protons - 3.56 (“quartet”, 4H - next to O), 3.43 (“triplet”, next to N), 3.34 (“triplet”, next to N). Mass-spectrometry, positive FAB (Ar): for C7H10N2O2, calculated - 154.07 (found - 154.08).
References:
Riddles, Courtney N.;Whited, Mark;Lotlikar, Shalaka R.;Still, Korey;Patrauchan, Marianna;Silchenko, Svitlana;Gerasimchuk, Nikolay [Inorganica Chimica Acta,2014,vol. 412,p. 94 - 103]

110-89-4
0 suppliers
$15.96/100ML

372-09-8
368 suppliers
$20.00/25g

15029-30-8
81 suppliers
$12.00/1g

110-89-4
0 suppliers
$15.96/100ML

105-34-0
458 suppliers
$5.00/25g

15029-30-8
81 suppliers
$12.00/1g