
1-Cyclohexanesulfonyl-piperazine synthesis
- Product Name:1-Cyclohexanesulfonyl-piperazine
- CAS Number:1183873-04-2
- Molecular formula:C10H20N2O2S
- Molecular Weight:232.34
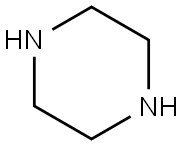
110-85-0
553 suppliers
$5.00/5G
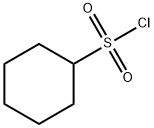
4837-38-1
81 suppliers
$18.00/500mg

1183873-04-2
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 4 h;
Steps:
General procedure for preparation of sulfonamide EA derivatives
General procedure: The synthesis of the key intermediates compounds 2(a-i), 3(a-i) as well as 4(a-i) were achieved following the previously reported procedures. Ethylene diamine sulfonyl 2(a-i) and piperaziene sulfonyl 3(a-i) were prepared according to references 48-53, while, the 4-aminopiperidine-1-sulfonyl 4(a-i) were prepared according to reference 55. To a solution of 1,2-diaminoethane or piperazine (5.00 mmol) in dichloromethane (10 mL), triethylamine (1.51 g, 2 mL, 15.00 mmol) were added with stirring. The mixture was cooled to 0°C and a solution of the appropriate sulfonyl chloride (2.50 mmol) in dichloromethane (2 mL) was added dropwise. The mixture was stirred at room temperature for 4 hours. The reaction mixture was washed with 20 mL saturated aqueous solution of NaHCO3 and the organic layer was extracted with ethyl acetate (3x 20mL), dried over magnesium sulfate and concentrated under reduced pressure to give the desired intermediates 2(a-i) and 3(a-i). For the synthesis of intermediates 4(a-i), a solution of 4-(N-Boc) aminopiperidine (0.10 g, 0.50 mmol) in dichloromethane (10 mL) and triethylamine (0.15 g, 0.2 mL, 1.50 mmol) was added at 0°C. Then, a solution of sulfonyl chloride (0.50 mmol) in dichloromethane (2 mL) was added in small portions. The reaction was stirred for 4 hours, then, the reaction mixture was quenched with 20 mL of a saturated aqueous solution of NaHCO3. The aqueous layer was extracted with ethyl acetate (3x 20mL), dried over magnesium sulfate and concentrated under reduced pressure. The crude product was dissolved in solution (5 mL) of HCl in dioxane at room temperature for 1h. All solvents were removed under reduced pressure, which gave the corresponding crude salt. The intermediates 2(a-i), 3(a-i) and 4(a-i) were used in the next step directly without further purification. To a solution of EDC (0.62 g, 0.39 mmol), HOBt (0.60 g, 0.39 mmol) and ethacrynic acid (0.10 g, 0.33 mmol) in dry DMF (5 mL) at 0°C, crude amine (2(a-i) or 3(a-i)) was added. In the case of the intermediates 4(a-i), 0.30 mmol of triethylamine was used and the solution was stirred at room temperature. The reaction was monitored by TLC until all the amine was consumed. The mixture was diluted with ethyl acetate (100 mL) and extracted (3x 20mL). The organic phase was washed with water (2 × 50 mL) and brine (3 × 50 mL), dried over anhydrous MgSO4 and concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography.
References:
Bignon, Jér?me;El Abbouchi, Abdelmoula;El Brahmi, Nabil;El Kazzouli, Sa?d;Guillaumet, Gérald;Hiebel, Marie-Aude;Suzenet, Franck [Bioorganic and medicinal chemistry letters,2020,vol. 30,# 19] Location in patent:supporting information