
1-CYCLOHEXYL-2,2,2-TRIFLUORO-ETHANONE synthesis
- Product Name:1-CYCLOHEXYL-2,2,2-TRIFLUORO-ETHANONE
- CAS Number:6302-04-1
- Molecular formula:C8H11F3O
- Molecular Weight:180.17
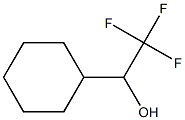
107018-38-2
9 suppliers
inquiry

6302-04-1
18 suppliers
$295.00/1g
Yield:-
Reaction Conditions:
with tetra(n-butyl)ammonium hydrogen sulfate in dichloromethane;water;
Steps:
25 Trifluoromethyl cyclohexyl ketone
EXAMPLE 25 Trifluoromethyl cyclohexyl ketone 10.67 g (0.059 mol) of 2,2,2-trifluoro-1-cyclohexylethanol and 0.98 g (0.003 mol) of tetrabutylammonium hydrogen sulfate are dissolved in 250 ml of methylene chloride at room temperature. 37 ml (0.069 mol) of an approximately 12% strength sodium hypochlorite solution are metered in within 20 minutes with vigorous stirring and the mixture is stirred for a further 4 hours during which the reaction temperature rises to 30° C. The reaction mixture is added to 200 ml of water, the phases are separated, the aqueous phase is extracted several times with methylene chloride and the combined organic phases are washed with saturated aqueous sodium chloride solution. After drying the organic phase with sodium sulfate, the volatiles are distilled off. Following distillation of the crude product (28 torr, 80°-82° C.), 4.86 g (46% of theory) of trifluoromethyl cyclohexyl ketone are obtained. nD20: 1.4043.
References:
US5608062,1997,A
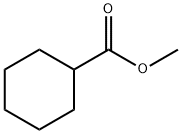
4630-82-4
197 suppliers
$6.00/25g
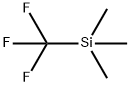
81290-20-2
410 suppliers
$13.00/1g

6302-04-1
18 suppliers
$295.00/1g
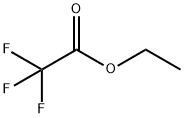
383-63-1
478 suppliers
$10.00/25g
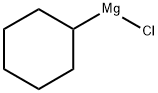
931-51-1
127 suppliers
$45.25/100ml

6302-04-1
18 suppliers
$295.00/1g
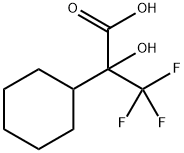
502607-34-3
1 suppliers
inquiry

6302-04-1
18 suppliers
$295.00/1g
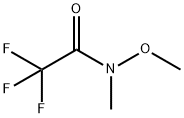
104863-67-4
146 suppliers
$5.00/250mg
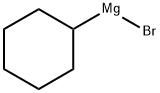
931-50-0
109 suppliers
$45.00/1g

6302-04-1
18 suppliers
$295.00/1g