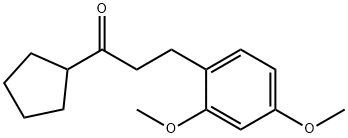
1-CYCLOPENTYL-3-(2,4-DIMETHOXYPHENYL)PROPAN-1-ONE synthesis
- Product Name:1-CYCLOPENTYL-3-(2,4-DIMETHOXYPHENYL)PROPAN-1-ONE
- CAS Number:625445-85-4
- Molecular formula:C16H22O3
- Molecular Weight:262.34
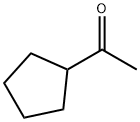
6004-60-0
137 suppliers
$25.30/250mg
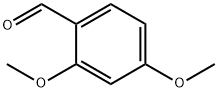
613-45-6
483 suppliers
$10.00/5g

625445-85-4
9 suppliers
$215.00/1g
Yield:625445-85-4 41%
Reaction Conditions:
Stage #1: 1-cyclopentylethanone;2,4-Dimethoxybenzaldehydewith sodium hydroxide in ethanol;water at 20; for 18 h;
Stage #2: with hydrogen;platinum(IV) oxide in ethyl acetate at 20;
Steps:
B.86.1 Step 1: 3- [2- (2, 4-DIMETHOXYPHENYL) ETHYL]-1-CYCLOPENTYLPROPAN-1-ONE
A solution OF 2, 4-DIMETHOXYBENZALDEHYDE (10.27 g, 45 mmol) and methyl cyclopentyl ketone (6.06 g, 54 mmol) in anhydrous ethanol (81 mL) was treated with 5 M NAOH (aq) (18 mL, 90 mmol) and the mixture stirred at room temperature for 18 h. The volatiles were removed in vacuo. The residue was extracted with ether (100 mL) and the extract washed with water (3 x 60 mL), then with brine. The ethereal solution was dried over MGS04, filtered, and concentrated in vacuo, affording the intermediate chacone in a crude yield of 14.63 g. The crude intermediate (14.52 G) was dissolved in 110 mL ethyl acetate, treated with platinum oxide (5 mole%) and stirred over 1 atm of H2 at room temperature overnight. The Pt was filtered through a fine fritted funnel and the black residue washed with ethyl acetate. The filtrate was concentrated in vacuo to give a yellowish resin. The resin was chromatographed using silica gel and 6: 1 HEXANES/ETHYL acetate, yielding 6.02 g (41 %) of the ketone as a colorless oil. H NMR (CDC13) : 5 1.48-1. 81 (m, 8H), 2.67 (m, 2H), 2.80 (m, 3H), 3.76 (s, 3H), 3.75 (s, 3H), 6.37 (dd, 1 H, J= 8.1, 2.1 Hz), 6.41 (d, 1H, J= 2.1 Hz), 7.00 (d, 1H, J= 8.1 Hz). MS (APCI) calcd for C6H2203 : 262.2 ; found (M+H) : 263.1.
References:
WO2004/74270,2004,A2 Location in patent:Page 336-337