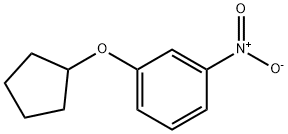
1-Cyclopentyloxy-3-nitro-benzene synthesis
- Product Name:1-Cyclopentyloxy-3-nitro-benzene
- CAS Number:1031442-10-0
- Molecular formula:C11H13NO3
- Molecular Weight:207.23
Yield:-
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in tetrahydrofuran at 20; for 4 h;Inert atmosphere;Cooling with ice;Mitsunobu Displacement;
Steps:
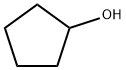
1-(Cyclopentyloxy)-3-nitrobenzene (93)
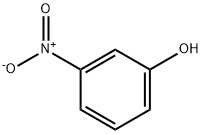
To a solution of 3-nitrophenol (0.14g, 1 mmol) in THF (5 ml) was added triphenylphosphine (0.29 g, 1.1 mmol), cyclopentanol 0.1 ml, 1.1 mmol) under nitrogen. The reaction was cooled in an ice- water bath and diisopropyl azodicarboxylate (0.22 ml, 1.1 ml) was added slowly over10 mm. The reaction was raised to room temperature and stirred for 4 h. After removal of solvent. The reaction mixture was concentrated under reduced pressure and purified by column chromatography (5i02, ethyl acetate/hexanes) to provide the desired product as white solid (0.14 g, 70%). 1 H NMR (300 MHz, ODd3) O 7.77 (ddd, J = 0.94, 2.07, 8.10 Hz, 1H), 7.69 (t, J = 2.35 Hz, 1H), 7.40 (t, J = 8.19 Hz, 1H), 7.18(ddd, J = 0.75, 2.45, 8.29 Hz, 1H), 4.83 (tt, J = 2.61, 5.58 Hz, 1H), 1.76-2.03 (m,6H), 1.61-1.72 (m, 2H). MS(ESl) m/zforC11H13NO3[M+H]+: calcd: 208.1; found:208.2.
References:
RTI INTERNATIONAL;ZHANG, Yanan;NGUYEN, Thuy;GERMAN, Nadezhda WO2018/209030, 2018, A1 Location in patent:Paragraph 71; 124