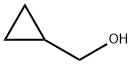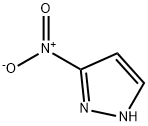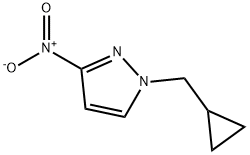
1-Cyclopropylmethyl-3-nitro-1H-pyrazole synthesis
- Product Name:1-Cyclopropylmethyl-3-nitro-1H-pyrazole
- CAS Number:1003013-15-7
- Molecular formula:C7H9N3O2
- Molecular Weight:167.17
Yield:1003013-15-7 58%
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in tetrahydrofuran; for 0.5 h;
Steps:
69
The 3-Nitro-1H-pyrazole (prepared in example 3, 255 mg, 2.26 mmol) was dissolved in tetrahydrofuran (11 mL) and cyclopropyl-methanol (163 mg, 2.26 mmol) was added followed by triphenylphosphine (592 mg, 2.26 mmol). While stirring, diisopropyl azodicarboxylate (667 μL, 3.39 mmol) was added dropwise. A mild exotherm was observed. The reaction continued to stir for 30 min. The solution was diluted with ethyl acetate (100 mL), washed with water (2×35 mL), 1.0 M aqueous hydrochloric acid solution (35 mL), saturated aqueous brine solution (35 mL), dried over magnesium sulfate, filtered and concentrated in vacuo. Purification by flash column chromatography (Merck silica gel 60, 40-63 μm; 5% ethyl acetate/hexanes to 50% ethyl acetate/hexanes) afforded 1-cyclopropylmethyl-3-nitro-1H-pyrazole (217 mg, 58%) as a yellow oil: H1-NMR (400 MHz, CDCl3) δ 0.44 (2H, qt, J=5.6 Hz), 0.73 (2H, qt, J=6.8 Hz), 1.30-1.40 (1H, m), 4.07 (2H, d, J=7.6 Hz), 6.89 (1H, d, J=2.8 Hz), 7.57 (1H, d, J=2.4 Hz).
References:
US2008/21032,2008,A1 Location in patent:Page/Page column 63