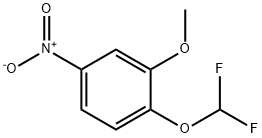
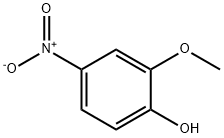
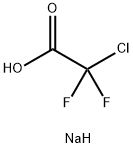
Yield:1198109-79-3 67%
Reaction Conditions:
with caesium carbonate in water;N,N-dimethyl-formamide at 20 - 80; for 62 h;Inert atmosphere;
Steps:
36
Preparation 36 1-(Difluoromethoxy)-2-methoxy-4-nitrobenzene; 4-Nitro-2-methoxyphenol (2.00 g, 11.8 mMol) was added to dimethylformannide (100 mL) and water (6.3 imL), that had been degassed by passage of argon (g) for 30 minutes. To this stirred solution was added sodium difluorochloroacetate (5.41 g, 35.5 mMol) followed by caesium carbonate (28.9 g, 88.3 mMol) in one portion. The reaction mixture was then heated to 8O 0 C for 24 hours. The reaction mixture was allowed to stand at room temperature for a further 38 hours and was then diluted with EtOAc (50 mL) and washed with water (3 x 20 mL). The organic layer was dried over magnesium sulphate, filtered and concentrated in vacuo to give the title compound as a yellow oil (4.20 g, 67%).1H NMR (300MHz, CDCI3) δ = 3.98 (s, 3H), 6.66 (t, 2H), 7.26 (d, 1 H), 7.84-7.86 (m, 2H) LCMS (System 5): 2.2 mins
References:
WO2009/144632,2009,A1 Location in patent:Page/Page column 70-71