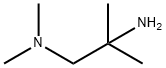
1-DIMETHYLAMINO-2-METHYL-2-AMINOPROPANE synthesis
- Product Name:1-DIMETHYLAMINO-2-METHYL-2-AMINOPROPANE
- CAS Number:89379-40-8
- Molecular formula:C6H16N2
- Molecular Weight:116.2
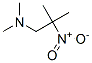
19155-54-5
0 suppliers
inquiry

89379-40-8
20 suppliers
$527.00/500mg
Yield:89379-40-8 76%
Reaction Conditions:
Stage #1: N,N-2-trimethyl-2-nitropropanaminewith hydrogenchloride in water; for 0.0333333 h;Cooling with ice;
Stage #2: with zinc in water at 20;
Steps:
INTERMEDIATE 11 N1,N1-trimethylpropane-1,2-diamine
INTERMEDIATE 11 N1,N1-trimethylpropane-1,2-diamine To ice cold cone. HC1 (30 mL, 360 mmol) was added N,N,2-trimethyl-2-nitropropan-l -amine (INTERMEDIATE 10, 4.0 g, 27.6 mmol). The mixture was stirred for 2 min and then Zn (10 g, 152 mmol) was added in small portions over 45 minutes. Initially the mixture turned white- cloudy. When approximately 60% of the Zn was added, the mixture stayed metal-gray. After all of the Zn was added the mixture was stirred overnight at room temperature. The reaction mixture was cooled with an ice bath, and solid NaOH pellets were added in small portions until pH >12 was obtained. Water (10-20 mL) was added to the viscous mixture which was then extracted with diethyl ether (4x40 mL). The combined organic phases were dried over Na2SC"4, and the organic phase was added via a dropping funnel to a Claisen distillation apparatus. The ether was removed at a bath temperature of ca 55-60 °C. When all of the solvent was removed, the bath temperature was increased to 135-140 °C, and after a short fore run, 2.4 g (76%) of pure title product was collected at 117-122 °C. 1H NMR (600 MHz, CDC13) δ ppm 2.33 (s, 6 H) 2.18 (s, 2 H) 1.91 (br. s., 2 H) 1.07 (s, 6 H). MS (ESI+) m/z 117 [M+H]+.
References:
WO2016/124553,2016,A1 Location in patent:Page/Page column 75
62983-26-0
15 suppliers
$220.00/0.5g

89379-40-8
20 suppliers
$527.00/500mg