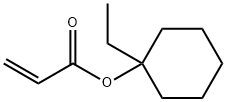
1-Ethyl-1-cyclohexyl acrylate synthesis
- Product Name:1-Ethyl-1-cyclohexyl acrylate
- CAS Number:251909-25-8
- Molecular formula:C11H18O2
- Molecular Weight:182.26

74-96-4
430 suppliers
$9.00/5g

108-94-1
530 suppliers
$12.00/50g

814-68-6
380 suppliers
$21.21/5gm:

251909-25-8
46 suppliers
inquiry
Yield:251909-25-8 74.1%
Reaction Conditions:
Stage #1: ethyl bromidewith magnesium in tetrahydrofuran;toluene at 13 - 30; for 2 h;Inert atmosphere;
Stage #2: cyclohexanone in toluene at 60; for 2.5 h;Inert atmosphere;
Stage #3: acryloyl chloridewith triethylamine in 1,2-dichloro-ethane at 55 - 70; for 1.5 h;Inert atmosphere;Solvent;Temperature;Reagent/catalyst;
Steps:
1-4 Preparation of 1-ethylcyclohexyl acrylate:
Weigh 6.2g (0.26mol) magnesium shavings in 500mlThe three-necked flask was protected by dry nitrogen. Weigh 32.0g (0.296mol) of bromoethane,46.0g of tetrahydrofuran and 60.0g of toluene were mixed evenly and placed in a constant pressure dropping funnel,The internal temperature is 13 ° C. MagnesiumAdd 5mL of a mixture of tetrahydrofuran, toluene and bromoethane,After the reaction was initiated, a lot of heat was released,The internal temperature rose to 35 ° C, the solution turned gray and white smoke came out.When the internal temperature drops to 15 , the continuous dropwise addition of ethyl bromide,Mixture of tetrahydrofuran and toluene and start stirring.Control internal temperature <35 , 1h dropAfter completion, transfer to room temperature (20-30 ) and stir for 1 hour to prepare Grignard reagent.In addition, 19.6g (0.2mol) cyclohexanone was dissolved in 20.0g toluene and placed in a constant pressure dropping funnel, and the dropwise addition to the previously prepared Grignard reagent was started.The reaction system emits heat obviously, and the internal temperature is controlled to be less than 60 There was slight reflux in the reaction system. After 1.5h,After holding at 60 for 1h,The reaction system was cooled to 55 ,Add 20.0g of triethylamine to it; Weigh 27.0g (0.3mol) of acryloyl chloride and65.0g of dichloroethane is mixed evenly and placed in 100mlConstant pressure dropping funnel.When the internal temperature of the reaction system is 55 ,Start dropping the mixture of acryloyl chloride and dichloroethane,With the addition of the mixed solution,The reaction slowly exothermed, and the internal temperature was controlled to drop below 70 ° C.After 30 minutes of dropping, the reaction was stopped after stirring at 70 for 1 hour,After cooling to 25 ° C, the reaction solution was poured into 500ml of water and stirred for 30min.Separate the liquid and extract the aqueous phase with 150.0 g × 2 of dichloroethane,The organic phases were combined, washed with 100.0g × 2 water, dried with 50.0g anhydrous sodium sulfate, filtered with suction, and rotary evaporated.36.0 g of crude product was obtained in a dark brown viscous state with a GC purity of 88.7% and a yield of 98.8%.Distill the crude product with an oil pump under reduced pressure, add 0.001g of p-methoxyphenol to the distillation bottle at 60PaCollect (98-100 ) fractions to obtain 27.0g of product as a colorless transparent liquid with good fluidity.The GC purity is 99.58% and the yield is 74.1%.
References:
CN104910012,2016,B Location in patent:Paragraph 0028-0055