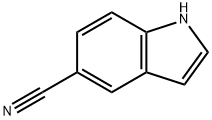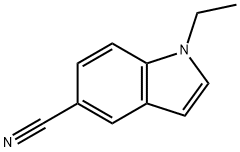
1-ethyl-1H-indole-5-carbonitrile synthesis
2synthesis methods
- Product Name:1-ethyl-1H-indole-5-carbonitrile
- CAS Number:83783-28-2
- Molecular formula:C11H10N2
- Molecular Weight:170.21
Yield:83783-28-2 88%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide;mineral oil at 20 - 40; for 2 h;
Steps:
1.A Step A 1-ethyl-1H-indole-5-carbonitrile (II)
Add 100.2g (5.0mol) 60% NaH to 1300mLN,N-dimethylformamide at room temperature,Stir, slowly warm up to 40°C, add 5-cyanoindole 284.0g (2.0mol), after H2 escapes, cool to 25°C, add ethyl iodide 468.0g (3.0mol), react at room temperature for 2h. After the reaction was completed, 5000 mL of water was added, and after stirring for 30 min, 299.2 g of solid was obtained by suction filtration, with a yield of 88.0%.
References:
CN111187261,2020,A Location in patent:Page/Page column 32