1-ethyl-3-(2-hydroxyethyl)urea synthesis
- Product Name:1-ethyl-3-(2-hydroxyethyl)urea
- CAS Number:29346-51-8
- Molecular formula:C5H12N2O2
- Molecular Weight:132.16
Yield:29346-51-8 65%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 0 - 20;
Steps:
60.1
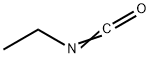
Example 60 l-(2-((2-amino-5-(2-(difluoromethyl)-6-methylpyridin-4-yl)-6-(4-fluorophenyl)pyrimidin-4- yl)oxy)ethyl)-3-ethylurea [00356] Step 1: l-ethyl-3-(2-hydroxyethyl)urea. To a mixture of 2-aminoethanol (2.0 g, 32.7 mmol) in DCM (100 mL) was added DIPEA (8.45 g, 65.5 mmol) and isocyanatoethane (3.03 g, 42.6 mmol) at 0 °C. After stirring for 30 min at 0 °C, the reaction mixture was warmed slowly to room temperature, then stirred overnight. The reaction mixture was concentrated and the residue was purified by reverse phase silica gel column to get l-ethyl-3-(2-hydroxyethyl)urea (2.8 g, 65%) as a colorless oil.
References:
WO2020/14332,2020,A1 Location in patent:Paragraph 00356