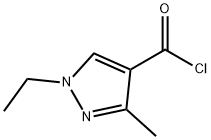
1-ethyl-3-methyl-1H-pyrazole-4-carbonyl chloride synthesis
- Product Name:1-ethyl-3-methyl-1H-pyrazole-4-carbonyl chloride
- CAS Number:1171573-50-4
- Molecular formula:C7H9ClN2O
- Molecular Weight:172.61
113131-46-7
36 suppliers
$45.00/10mg

1171573-50-4
9 suppliers
$359.00/500mg
Yield:-
Reaction Conditions:
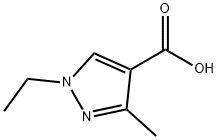
with thionyl chloride at 79; for 3 h;
Steps:
339.1
1-Ethyl-3-methyl-1H-pyrazole-4-carboxylic acid [3-(3-hydroxymethylene-2-oxo-2,3-dihydro-1H-indole-6-carbonyl)-phenyl]-amide was prepared from 3-nitrobenzoyl chloride using the following multiple step procedure: Step 1: 2-Phenyl-2H-pyrazole-3-carboxylic acid [3-(2-oxo-2,3-dihydro-1H-indole-6-carbonyl)-phenyl]-amide.; A dry flask was charged with 1-Ethyl-3-methyl-1H-pyrazole-4-carboxylic acid (0.122 g, 0.791 mmol) and thionyl chloride (5 mL) and allowed to stir at 79° C. for 3 h. The thionyl chloride was then removed by concentration in vacuo. The crude acid chloride was cooled to room temperature, and then dissolved in THF (8 mL). 6-(3-Amino-benzoyl)-1,3-dihydro-indol-2-one (as prepared in Example 40, 0.200 g, 0.793 mmol) was added to the THF solution of the acid chloride, and the mixture was allowed to reflux overnight. The reaction mixture was then allowed to cool to room temperature and subsequently vacuum filtered. The solid was washed with 0° C. THF (5 mL) and collected to afford the 2-Phenyl-2H-pyrazole-3-carboxylic acid [3-(2-oxo-2,3-dihydro-1H-indole-6-carbonyl)-phenyl]-amide as a solid (0.249 g, 0.640 mmol, 81%).
References:
US2007/32478,2007,A1 Location in patent:Page/Page column 149