
1-ETHYL-4-HYDROXY-6-METHYLPYRIDIN-2(1H)-ONE synthesis
- Product Name:1-ETHYL-4-HYDROXY-6-METHYLPYRIDIN-2(1H)-ONE
- CAS Number:61296-13-7
- Molecular formula:C8H11NO2
- Molecular Weight:153.18
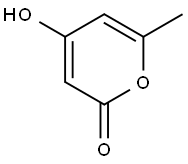
675-10-5
232 suppliers
$9.00/1g
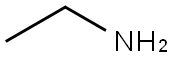
75-04-7
376 suppliers
$10.00/20mg

61296-13-7
18 suppliers
$130.00/100mg
Yield:61296-13-7 79%
Reaction Conditions:
in water at 100;
Steps:
Representative procedure for the preparation of pyridones 6a-6g
A mixture of triacetic acid lactone (1) (0.5g, 3.96 mmol, 1equiv) and primary amine (4.35mmol, 1.1equiv) in water (2.5mL) was heated to 100°C overnight. After completion of the reaction (TLC monitoring), the reaction mixture was cooled down and the precipitate was filtered and washed with ethyl acetate and dried under vacuum to obtain the desired product.121-Ethyl-4-hydroxy-6-methylpyridin-2(1H)-one (6a) (79%) Light brown solid: mp 240-242°C (lit. 247°C)6; Rf=0.16 (silica gel, CH2Cl2/EtOAc 1:1); 1H NMR (300MHz, methanol-d4) δ=5.92 (d, 1H), 5.72 (d, 1H), 4.05 (q, 2H), 2.39 (s, 3H), 1.24 (t, 3H) ppm; 13C NMR (126MHz, methanol-d4) δ=165.74, 164.21, 145.70, 100.64 (2C), 37.09, 17.14, 11.18ppm; LRMS (ESI-QTOF) calcd for C8H12NO2 [M+H]+ 154.0868, found 154.0872.
References:
Kraus, George A.;Wanninayake, Umayangani K.;Bottoms, Jashaun [Tetrahedron Letters,2016,vol. 57,# 11,p. 1293 - 1295]