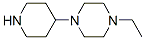
1-ETHYL-4-PIPERIDIN-4-YL-PIPERAZINE synthesis
- Product Name:1-ETHYL-4-PIPERIDIN-4-YL-PIPERAZINE
- CAS Number:435341-92-7
- Molecular formula:C11H23N3
- Molecular Weight:197.32
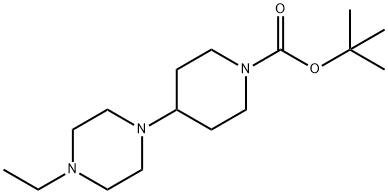
1037238-83-7
0 suppliers
inquiry
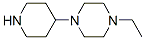
435341-92-7
23 suppliers
inquiry
Yield:435341-92-7 87%
Reaction Conditions:
with hydrogenchloride in methanol;dichloromethane;isopropyl alcohol at 20 - 40; for 50 h;Reflux;
Steps:
a.a.2
a.2) 1-Ethyl-4-piperidin-4-ylpiperazine as chloride salt The protective group was removed by introducing 40 g (135 mmol) of tert-butyl 4-(4-ethylpiperazin-1-yl)piperidine-1-carboxylate from example a.1 into 200 ml of methanol and 1.8 l of dichloromethane and adding 100 ml of 5-6M HCl solution in isopropanol. A suspension resulted, and slight gas evolution was also observable. The reaction mixture was stirred at 40° C. (water bath temperature) for one hour and then stirred at room temperature for 48 hours. For complete deprotection, 50 ml of the 5-6M HCl solution in isopropanol were again added and the reaction mixture was stirred at 40° C. The dichloromethane was distilled out in a rotary evaporator. 200 ml of methanol and 30 ml of the 5-6M HCl solution in isopropanol were again added. The reaction mixture was stirred under reflux for one hour, during which a white suspension formed with strong evolution of gas. A mobile suspension then resulted and was cooled to room temperature. The precipitate was filtered off with suction and washed with methanol and diethyl ether. After drying, 36 g (117 mmol, 87%) of 1-ethyl-4-piperidin-4-ylpiperazine were isolated as chloride salt. 1H-NMR (D2O, 400 MHz) δ[ppm]=3.74-3.47 (m, 11H), 3.28 (q, 2H, J=7.3 Hz), 3.06 (dt, 2H, J=2.2 Hz, J=13.2 Hz), 2.38 (m, 2H, J=13.6 Hz), 1.89 (dq, 2H, J=4.1 Hz, J=13.3 Hz), 1.30 (t, 3H, J=7.3 Hz).
References:
US2011/65720,2011,A1 Location in patent:Page/Page column 19
5308-25-8
444 suppliers
$15.00/5G
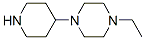
435341-92-7
23 suppliers
inquiry

79099-07-3
543 suppliers
$5.00/5g
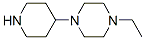
435341-92-7
23 suppliers
inquiry

5308-25-8
444 suppliers
$15.00/5G

79099-07-3
543 suppliers
$5.00/5g
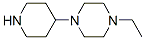
435341-92-7
23 suppliers
inquiry