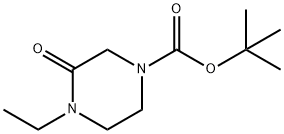
1-ETHYL-4-(TERT-BUTYLOXYCARBONYL)PIPERAZIN-2-ONE synthesis
- Product Name:1-ETHYL-4-(TERT-BUTYLOXYCARBONYL)PIPERAZIN-2-ONE
- CAS Number:194350-95-3
- Molecular formula:C11H20N2O3
- Molecular Weight:228.29
Yield:194350-95-3 81%
Reaction Conditions:
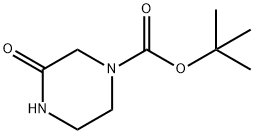
Stage #1: 4-(t-butyloxycarbonyl)piperazin-2-onewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.416667 h;
Stage #2: ethyl iodide in N,N-dimethyl-formamide at 40; for 15 h;
Steps:
40.2 2)
2) 4-Ethyl-3-oxopiperazine-1-carboxylic acid tert-butyl ester The above 3-oxopiperazine-1-carboxylic acid tert-butyl ester (9.36 g) was dissolved in N,N-dimethylformamide (105 mL), and, at 0°C, sodium hydride (1.361 g) was added to the solution, followed by stirring for 25 minutes. Ethyl iodide (4.48 mL) was added to the reaction mixture, and the resultant mixture was stirred for 15 hours at 40°C, followed by cooling in air. The reaction mixture was partitioned between water and ethyl acetate, and the aqueous layer was extracted with ethyl acetate. The organic layers were combined, washed with saturated brine, and dried over sodium sulfate anhydrate. After a filtration step, the solvent was evaporated under reduced pressure, and the residue was purified through silica gel column chromatography (chloroform - acetone), to thereby give 4-ethyl-3-oxopiperazine-1-carboxylic acid tert-butyl ester as an oily product (8.69 g, 81%). 1H-NMR(400MHz,CDCl3)δ:1.15(3H,t,J=7.1Hz), 1.46(9H,s), 3.33(2H,t,J=5.4Hz), 3.46(2H,q,J=7.1Hz), 3.64(2H,t,J=5.4Hz), 4.06(2H,s). EI-MS m/z:228(M+).
References:
EP1762568,2007,A1 Location in patent:Page/Page column 57

24424-99-5
823 suppliers
$13.50/25G

194350-95-3
7 suppliers
inquiry